Abstract
Introduction
Upper extremity deep vein thrombosis (UEDVT) represents up to 10% of cases of venous thromboembolism (VTE) and is frequently associated with central venous catheter (CVC) placement in patients receiving chemotherapy for cancer. UEDVT may be treated with low molecular weight heparin (LMWH) either as monotherapy or subsequently transitioned to warfarin as we have previously shown (Kovacs 2007). Whereas for non-cancer VTE rivaroxaban is at least as efficacious and safe as warfarin, the latter is problematic in cancer patients and direct oral anticoagulants (DOACs) such as rivaroxaban have not been studied to date in this setting. In this study weevaluated thesafety and efficacy of rivaroxaban in the treatment of UEDVT secondary to CVC in patients with cancer.
Methods
We conducted a multicentre prospective cohort study at 3 centres in Canada between December 2012 and January 2016. We enrolled patients ≥18 years of age with active malignancy and symptomatic proximal UEDVT (axillary or more proximal) with or without pulmonary embolism (PE), associated with a CVC. Exclusion criteria included dialysis catheters, active bleeding, platelet count <75 x 109/L, creatinine clearance <30 mL/min, other anticoagulants, PE with hemodynamic instability, inability to infuse through the catheter after a trial of intraluminal thrombolytic therapy (tissue plasminogen activator,tPa), patients with acute leukemia, patients with multiple myeloma awaiting bone marrow transplant within 3 months, thrombosis involving the brachial,basilic or cephalic veins only, treatment for >7 days with other anticoagulant, need for dual antiplatelet therapy (recent stent), or concomitant use of P-glycoprotein and CYP3A4 inhibitors. Primary objective was an estimate of the proportion of catheter survival at 3 months, defined as infusion failure that does not respond to 2 mg oftPa. Secondary objectives included recurrence of DVT, PE, major bleeding, clinically relevant non-major bleeding (CRNMB) and death. All events were independently adjudicated. Patients were treated with rivaroxaban at a dose of 15 mgpo bid for 3 weeks, followed by 20 mgpo daily for 9 more weeks (minimum 12 weeks).tPa (oralteplase) for management of blocked lines was allowed. Patients were followed clinically for 12 weeks to assess for clinical events including recurrent DVT and/or PE, major bleeding and CRNMB, and by phone at 6 months.
Results
We included 70 patients (47[67%] women) with a mean age of 54.1 years. DVTs were diagnosed by ultrasound in 68 (97%) patients, and most commonly involved the subclavian (n=55, 79%) and axillary (n=49, 70%) veins, followed by the internal jugular, brachial, brachiocephalic and external jugular veins. Peripherally inserted central catheters (PICC) were most common (n=54, 77%), followed by port-a-cathlines (n=16, 23%). Types of active malignancy included breast (n=29, 41%), colon (n=8, 11%), colorectal (n=5, 7%), rectal (n=3, 4%), prostate (n=1, 1%), and other (n=24, 34%).
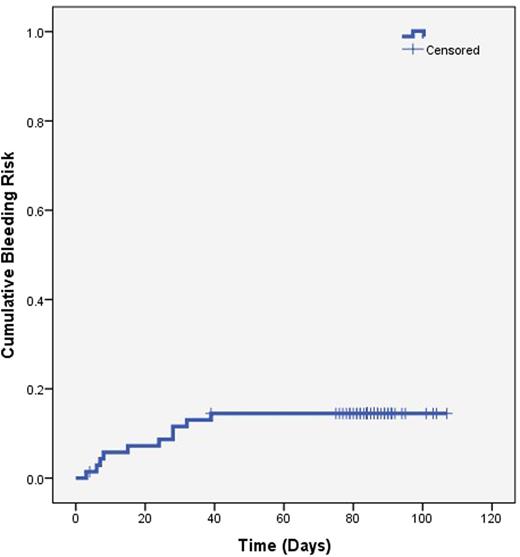
Catheter survival was 58.6% (95% CI 46.9 to 69.4) at 12 weeks and no catheters were removed due to thrombosis. Patients had their CVCs removed prior to the end of the study due to end of therapeutic need (n=20), infection (n=2), bleeding (n=2), kinking (n=2), patient preference (n=2), and death (n=1). The 3-month incidence rate of recurrent VTE was 1.43% (95% CI 0.25 to 7.66). There was 1 episode of recurrent VTE presenting as a fatal PE at 6 weeks. It was not known if the patient had a concurrent leg DVT at the time of the PE. There were no other deaths from any cause during the study. There were 11 bleeding events in 9 patients (12.85%, 95%CI 6.9 to 22.7), 6 major and 5 CRNMB (Figure 1). All bleeding events happened during the first 39 days of treatment. 7 patients discontinued anticoagulation during the study due to death (n=1), patient or clinician preference (n=5) and dermatological adverse reaction (n=1).
Discussion
In this study rivaroxaban showed promise in treating CVC-associated UEDVT in cancer patients, resulting in preserved CVC function. However, the bleeding rates and the occurrence of 1 death due to pulmonary embolism is concerning since we cannot exclude a causative role for the known UEDVT. Further studies are still required prior to recommending rivaroxaban in this setting.
Lazo-Langner:Daiichi Sankyo: Research Funding; Bayer: Honoraria; Pfizer: Honoraria. Tagalakis:Bayer: Honoraria. Louzada:Celgene: Consultancy, Honoraria; Bayer: Honoraria; Pfizer: Honoraria; Janssen: Consultancy, Honoraria. Kovacs:Bayer: Honoraria, Research Funding; Daiichi Sankyo Pharma: Research Funding; LEO Pharma: Honoraria; Pfizer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal