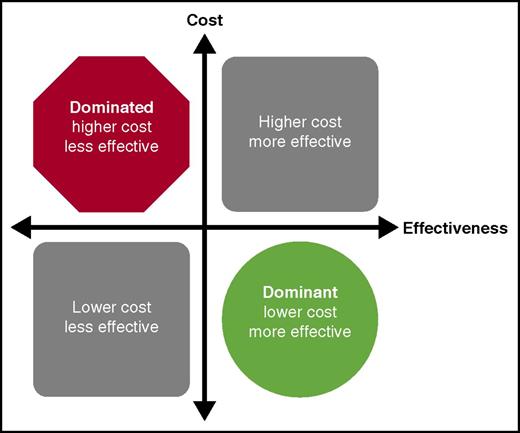
The cost-effectiveness of any intervention can be classified into 1 of 4 areas. If the treatment strategy is more effective and costs less than the comparison, it falls into the right lower quadrant and is referred to as the dominant strategy, indicating it should be the preferred strategy. When the strategy under consideration is less effective and costs more than the comparison, it falls into the upper left quadrant and is considered to be dominated by the other strategy. Interventions that fall into this quadrant should not be chosen when given the choice. The other 2 quadrants indicate tradeoffs between cost and effectiveness of the intervention and need to be considered in the context of other policy and clinical practice guideline decisions.
The cost-effectiveness of any intervention can be classified into 1 of 4 areas. If the treatment strategy is more effective and costs less than the comparison, it falls into the right lower quadrant and is referred to as the dominant strategy, indicating it should be the preferred strategy. When the strategy under consideration is less effective and costs more than the comparison, it falls into the upper left quadrant and is considered to be dominated by the other strategy. Interventions that fall into this quadrant should not be chosen when given the choice. The other 2 quadrants indicate tradeoffs between cost and effectiveness of the intervention and need to be considered in the context of other policy and clinical practice guideline decisions.
Cost-effectiveness analyses use analytic models to attempt to correlate the potential efficacy of a treatment and the associated costs with various outcomes.4 In each simulation, a hypothetical “patient” experiences one of various outcomes based on probabilities taken from the literature; in the absence of such data, outcomes are based on assumed values made by the research team in the context of reasonable clinical experience. Based on the output from the model, a potential treatment strategy is classified in one of several ways (see figure). When a proposed intervention is more effective and saves money at the same time (dominant), or is less effective and costs more than the comparison strategy (dominated), the decision is clear. When the intervention is more effective, but costs more, this tradeoff must be considered in the context of other policy interventions when allocating finite health-budget resources or creating clinical guidelines.
In their analysis, Aljabri and colleagues use a decision tree to simulate a cohort of patients with suspected HIT who undergo treatment with 1 of 3 possible anticoagulants: argatroban, bivalirudin, or fondaparinux. The model incorporates the time that patients need anticoagulation while awaiting confirmation of diagnosis and simulates the probability that each patient will experience complications such as venous thromboembolism or major bleeding. Probabilities for each of these outcomes come from the published literature. As each simulated patient undergoes treatment, the model accounts for the costs associated with treatment and complications. Decision models allow researchers to ask the “what if” question without the need to complete a separate clinical study including the associated costs and significant time investment.
In various simulations, fondaparinux was superior to argatroban and bivalirudin in terms of cost ($151 vs $1250 and $1466, respectively). Although there were few adverse events in the simulation, primarily because of the low rates of adverse events in general during treatment of HIT, fondaparinux had the least adverse events, suggesting it was the dominant strategy when comparing the 3 anticoagulants with each other.
Due to a lack of adequate published data to estimate the probabilities in each treatment group, the authors do not include other adverse outcomes such as amputation and death. This highlights a major limitation of decision models. The model is completely dependent on the inputs and assumptions made by those building the model, and the results could be different if a different set of event probabilities and assumptions are used.5
By varying an individual variable over a range of possible values, decision models also permit researchers to quantify how sensitive the model is to input uncertainty. In identifying those variables where a different input might have changed the model’s results, researchers and policy makers can outline priority areas for future research. The authors of this analysis looked at differences in institutional costs as well as the average wholesale price of the medications along with duration of therapy and differences in the incidence of HIT. In each case, fondaparinux remained the preferred treatment strategy. What remains to be seen is whether the cost-effectiveness of fondaparinux will change when compared with direct oral anticoagulants. Recent literature suggests these agents have efficacy and safety in the treatment of HIT, although larger clinical trials still need to be completed.6
Ultimately, cost-effectiveness analyses are not meant to dictate what should be done in clinical practice, but can inform clinical decision making as well as policy decisions at both institutional and governmental levels. The work by Aljabri and colleagues provides an important piece of information as health systems, governmental agencies, health payers, and medical societies make decisions about the most cost-effective way to manage HIT.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal