In this issue of Blood, Hamon et al reveal the existence of a marginated pool of inflammatory monocytes and demonstrate that migration of these cells to inflammatory sites is restricted by CX3C-chemokine receptor 1 (CX3CR1).1
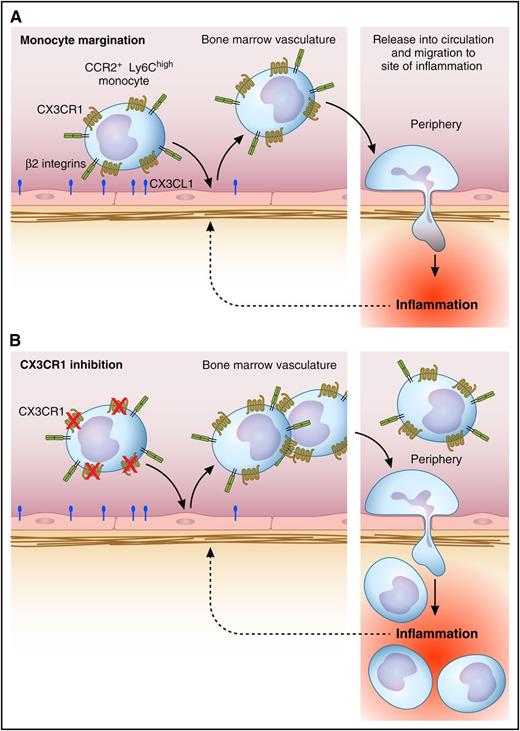
Regulation of margination of inflammatory monocytes in the bone marrow vasculature by CX3CR1. (A) Under steady-state conditions, inflammatory monocytes are attached to the endothelial surface within the bone marrow vasculature, a response partly dependent on CX3CR1. Upon induction of peritoneal inflammation, inflammatory monocytes undergo demargination and are rapidly released into the circulation, resulting in increased numbers in the circulation. This facilitates their recruitment to the peripheral site of inflammation via adhesion and transmigration in the microvasculature at the affected site. (B) Under conditions where CX3CR1 is inhibited or absent, the number of monocytes released into the circulation is greater, as is the level of monocyte recruitment to the periphery. Professional illustration by Patrick Lane, ScEYEnce Studios.
Regulation of margination of inflammatory monocytes in the bone marrow vasculature by CX3CR1. (A) Under steady-state conditions, inflammatory monocytes are attached to the endothelial surface within the bone marrow vasculature, a response partly dependent on CX3CR1. Upon induction of peritoneal inflammation, inflammatory monocytes undergo demargination and are rapidly released into the circulation, resulting in increased numbers in the circulation. This facilitates their recruitment to the peripheral site of inflammation via adhesion and transmigration in the microvasculature at the affected site. (B) Under conditions where CX3CR1 is inhibited or absent, the number of monocytes released into the circulation is greater, as is the level of monocyte recruitment to the periphery. Professional illustration by Patrick Lane, ScEYEnce Studios.
It is well recognized that neutrophils can rapidly increase in number in the circulation in response to various inflammatory stimuli. In the setting of infection, this is likely to be an important mechanism for controlling pathogen invasion.2 However, whether this applies to monocytes is less well understood. Seminal studies have demonstrated that circulating monocytes in mice and men are comprised of 2 main subsets: classical, also known as inflammatory, and nonclassical/patrolling. In addition to bearing different surface markers, these 2 populations are functionally distinct. Nonclassical monocytes migrate intravascularly, attached to the endothelial surface. From this location, they inspect the vascular microenvironment for signs of injury or infection and, if required, coordinate requisite inflammatory responses.3-5 In contrast, classical monocytes circulate in the bloodstream, from where they can undergo rapid recruitment to sites of inflammation and subsequently promote responses via generation of proinflammatory mediators.6,7 Monocyte numbers in the blood are controlled in an ongoing fashion by myelopoiesis and subsequent CCR2-dependent release from the bone marrow.8 However, studies from over 30 years ago indicated that, in response to some stimuli, the number of monocytes in the circulation can almost double within 10 minutes.9 The time course of this increase is clearly inconsistent with that of a response occurring solely via myelopoiesis. Were these additional monocytes derived from a previously unrecognized intravascular marginated pool?
Hamon et al addressed this issue using state-of-the-art imaging and flow cytometric approaches. Using 2-photon imaging, numerous monocytes were seen to patrol the lumen of the bone marrow vasculature. To characterize these cells more fully, Hamon et al used IV-administered antibodies to label intravascular cells and separate flow cytometric analysis of free cells in the circulation and those remaining attached in the tissue vasculature. Surprisingly, they found a large pool of inflammatory (CCR2+, Ly6Chigh) monocytes marginated in the bone marrow vasculature, in addition to nonclassical monocytes, identifying the bone marrow as a site of selective monocyte enrichment. Septic peritoneal inflammation resulted in a rapid reduction in the number of intravascular monocytes in the bone marrow. The number in the bloodstream also rapidly decreased in the first hour, but after 4 hours, it was significantly elevated above basal levels. In contrast, monocytes accumulated in the vasculature of the peritoneal lining, ultimately leading to the recruitment of macrophages into the peritoneal cavity (see figure). These results provided evidence of a redistribution of inflammatory monocytes from the bone marrow vasculature to the remote site of inflammation. Unexpectedly, in the absence of CX3CR1, both the monocytosis and the number of macrophages recruited to the peritoneal cavity were markedly elevated above levels in wild-type mice (see figure). Moreover, similar responses could be achieved via antagonism of this receptor using an inhibitory CX3CL1 analog. Interestingly, despite studies indicating that CX3CR1 expression is lower on inflammatory versus patrolling monocytes, inflammatory monocytes bound soluble CX3CL1 as effectively as patrolling monocytes. These findings indicate a previously unrecognized functional role for CX3CR1 on inflammatory monocytes in restricting their release into the bloodstream and migration into inflamed tissue. Furthermore, these findings show that this function can be blocked in real-time with a drug-like reagent.
Prior to this study, nonclassical monocytes were believed to be the major monocyte population patrolling the vascular endothelium. By extending the investigation of intravascular cells beyond those detectable in a straightforward blood sample, Hamon et al reveal that inflammatory monocytes represent a major proportion of the monocytes attached to the endothelium in the bone marrow microvasculature. Moreover, these cells can act as a reservoir of inflammatory monocytes available for rapid release into the circulation in response to local inflammation. However, release of this marginated pool of monocytes is held in check by the CX3CR1/CX3CL1 pathway. In an era where therapeutic uses of inhibiting this pathway are being explored, this study demonstrates that CX3CR1 antagonism can result in increased inflammation-associated monocytosis and increased delivery of macrophages to the target site. As such, in many inflammatory conditions, inhibition of this pathway may have unexpected inflammation-promoting effects associated with increased recruitment of inflammatory monocytes. However, in other settings, such as atherosclerosis, where the vasculature itself is the target of monocyte/macrophage-dependent inflammation, CX3CR1 inhibition reduces disease progression,10 indicating that this pathway remains a valid target in some circumstances. From these results, it is clear that implementation of CX3CR1 antagonism in inflammatory disease will need to be carefully considered, taking into account the potential to increase the number of inflammatory monocytes in the circulation, namely, the very cell type being targeted by the intervention.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal