In this issue of Blood, Sun et al demonstrate that replacing factor IX (FIX) for short periods of time does not prevent long-term arthropathy in a mouse model of hemophilia B.1
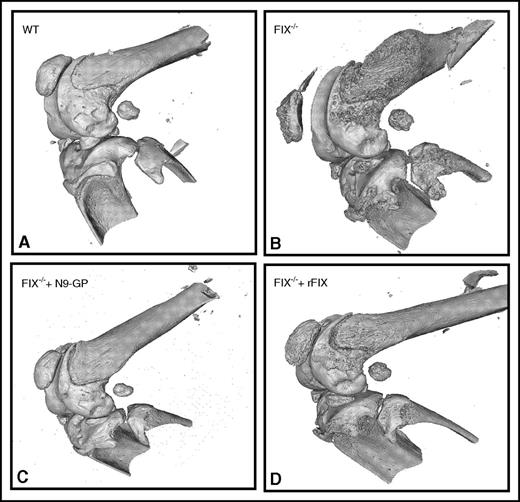
Abnormal bone wound healing using microCT in hemophilia B mice. In (A) hemostatically normal wild-type (WT), (B) untreated hemophilia B (FIX−/−), (C) hemophilia B treated with long-activing recombinant nonacog β pegol (N9-GP), or (D) unmodified rFIX mice. See Figure 4A in the article by Sun et al that begins on page 2161.
Abnormal bone wound healing using microCT in hemophilia B mice. In (A) hemostatically normal wild-type (WT), (B) untreated hemophilia B (FIX−/−), (C) hemophilia B treated with long-activing recombinant nonacog β pegol (N9-GP), or (D) unmodified rFIX mice. See Figure 4A in the article by Sun et al that begins on page 2161.
Hemophilia B is an X-linked bleeding disorder due to the deficiency of FIX. Recently, new recombinant FIX (rFIX) products with extended half-life (EHL) were approved for both prophylactic and on-demand therapy. In the work here, the authors compared a single injection of long-acting glycoPEGylated rFIX with the outcomes of multiple injections of unmodified rFIX in hemophilia B mice or hemostatically normal mice. In normal mice, the authors successfully demonstrate the limited effect of the induction of hemarthrosis, even when blood was injected into the joint, mimicking the phenotype of hemophilia B mice. EHL-FIX provides a superior effect in minimizing the joint disease in hemophilia B mice compared with single or multiple doses of unmodified FIX by 3 doses daily or 8 doses over 13 days. This was achieved despite comparable FIX levels in plasma and synovial fluid lavage at an early time point. Using a comprehensive histopathology and healing assessment of the synovial and osteochondral wound, they demonstrate that the single dose of EHL-FIX was superior to the multiple replacement strategy of unmodified FIX over time. Moreover, a single dose of EHL-FIX was comparable to the quick joint wound healing observed in hemostatically normal mice. Notably, iron deposition was remarkably reduced by a single dose of EHL-FIX. Interestingly, findings were also noted in the surrounding bone density and structure. The use of noninvasive microcomputed tomography (microCT) (see figure) also confirmed the superior performance of EHL-FIX.
Emerging evidence on the role of severity of intra-articular bleedings and its effect on bone tissue are also supported by these findings. Collectively, these data suggest that the standard clinical strategy of transient factor replacement for only a few days may stop acute bleeding, reduce pain, and improve parameters of joint mobility,2 but may not be enough to prevent the slow wound-healing process and resulting chronic arthropathy. These findings are supported by data from skin wound-healing studies showing delayed healing in hemophilia B mice compared with hemostatically normal mice.3,4 It will be interesting to explore whether replacement with EHL-FIX is superior to unmodified FIX in this wound-healing model. The underlying mechanism of the beneficial effect of EHL-FIX is unclear considering that the plasma half-life of the glycoPEGylated FIX is only ∼2.5 hours longer than unmodified FIX in this hemophilia B model. There is a possibility that binding of FIX to extravascular space was the reason for this long-term healing effect. It also may be possible that FIX extravascular binding may explain, at least in part, these effects because collagen IV, the main collagen form that binds to FIX, is present in the synovial intimal layer and meniscus.5,6 Interestingly, Stafford’s group suggested that most of the FIX may be located at extravascular sites upon protein infusion, and hemostasis can be achieved at late time points even if FIX is no longer detected in the circulation.7,8 These intriguing hypotheses inject some uncertainty regarding the clinical relevance of the typical pharmacokinetics of infused FIX as a marker of biological activity.
It will be interesting to know whether the beneficial effects demonstrated here with glycoPEGylated FIX can also be obtained using other forms of long-acting FIX or on long-term expression of FIX by gene therapy. One limitation of the study is the lack of a range of FIX doses tested to identify the minimal dosage with therapeutic effect. Here, they used a rather high fixed dose of FIX (250 IU/kg per injection), which would be economically challenging in a clinical setting.
Moreover, given the large effect on iron deposition demonstrated here, the use of magnetic resonance imaging of the joints could also be incorporated in clinical trials using EHL products to assess a potential protective effect. There are several reports on the use of EHL products in mouse models of hemophilia addressing hemostasis, immune responses, and now hemarthrosis; it will be interesting to see whether these findings have translational potential.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal