Key Points
Myeloid cell TF-dependent venous thrombosis is under control of PDI and the complement cascade.
C5 deficiency reduces fibrin formation and leukocyte PS exposure with normal platelet deposition in flow-restricted vessels.
Abstract
Expanding evidence indicates multiple interactions between the hemostatic system and innate immunity, and the coagulation and complement cascades. Here we show in a tissue factor (TF)–dependent model of flow restriction-induced venous thrombosis that complement factors make distinct contributions to platelet activation and fibrin deposition. Complement factor 3 (C3) deficiency causes prolonged bleeding, reduced thrombus incidence, thrombus size, fibrin and platelet deposition in the ligated inferior vena cava, and diminished platelet activation in vitro. Initial fibrin deposition at the vessel wall over 6 hours in this model was dependent on protein disulfide isomerase (PDI) and TF expression by myeloid cells, but did not require neutrophil extracellular trap formation involving peptidyl arginine deiminase 4. In contrast to C3−/− mice, C5-deficient mice had no apparent defect in platelet activation in vitro, and vessel wall platelet deposition and initial hemostasis in vivo. However, fibrin formation, the exposure of negatively charged phosphatidylserine (PS) on adherent leukocytes, and clot burden after 48 hours were significantly reduced in C5−/− mice compared with wild-type controls. These results delineate that C3 plays specific roles in platelet activation independent of formation of the terminal complement complex and provide in vivo evidence for contributions of complement-dependent membrane perturbations to prothrombotic TF activation on myeloid cells.
Introduction
The blood coagulation and complement systems are evolutionarily related enzymatic cascades contributing to host defense. Both systems control infection by pathogens, are connected in their activation mechanisms, and influence innate immune cell function following tissue injury.1 Genetic dispositions leading to hyperactive complement are increasingly recognized as contributors to vascular and thromboembolic diseases, including paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome,2 and antiphospholipid syndrome.3 The central role for complement is highlighted by the clinical efficacy of complement factor 5 (C5) inhibitors in preventing thrombotic complications in these diseases,4 but the connections between complement and hemostatic systems in other settings of thrombosis remain incompletely understood.
Proteolytic activation of C5 generates C5b that initiates the formation of the membrane attack complex. Even at sublytic concentrations, the terminal complement complex exposes negatively charged procoagulant phospholipids, and thereby may amplify coagulation reactions on platelets, leukocytes, and endothelial cells.5,6 Coagulation and fibrinolysis also conversely activate the complement system,7-10 leading to reciprocal amplification of the protease cascades that may be particularly important in sepsis.11 Platelets interact with the complement system and generate active complement components following platelet activation. Complement binding receptors, including P-selectin12 and C1q receptors,13 enable platelets to initiate the alternative or classical complement pathway.14 Classical complement pathway components are also found on procoagulant platelet-derived microparticles and may contribute to the efficiency of their clearance.13 Although C3 is crucial for platelet aggregation and C3−/− mice are protected from thrombosis,15 it is unknown whether complement makes contributions to thrombosis in vivo beyond directly activating platelets.
Circulating levels of activated C3 and activated C5 (C5a) are poorly correlated with thrombin-antithrombin levels in thrombosis models,8 indicating that amounts of thrombin generated are insufficient to directly induce complement activation. However, the demonstrated activation of complement on platelets raises the question whether complement may contribute to activation of coagulation in the setting of thrombosis. Although the complement product C5a can induce tissue factor (TF) expression in leukocytes,16,17 in vitro studies indicated that complement activation is particularly required for the rapid posttranslational conversion of monocyte-expressed TF to a fully procoagulant molecule.18 In this study, antibody-mediated complement activation was shown to cause the conversion of TF to a procoagulant form by simultaneously promoting C5-dependent cell surface thiol-disulfide exchange reactions involving protein disulfide isomerase (PDI), as well as phosphatidylserine (PS) exposure dependent on non-lytic C5b-C7 membrane insertion. These data suggested that complement is a relevant activator of thiol-disulfide reactions mediating the conformational activation of TF.19,20
Activated platelets release thiol isomerases and may therefore contribute to thiol-disulfide–dependent TF activation in thrombosis.21 PDI controls platelet integrin function, supports platelet-dependent thrombin generation, and leads to TF-dependent fibrin and thrombus formation at sites of vascular injury.22-26 Recent studies have shown that PDI binds to β3 integrins in vitro and in vivo.27 Anti-PDI antibodies prevent both thrombus formation and fibrin generation following vascular injury, suggesting that extracellular PDI could be a target for anti-thrombotic therapy.24,28,29 Genetic evidence furthermore shows that the second catalytic domain of PDI is crucial for platelet activation that also involves Erp57, another platelet-expressed thiol isomerase.30,31 Whether complement regulates platelet activation partially through thiol-disulfide exchange is currently unclear. Here we show that platelet activation and TF-dependent fibrin formation involve different stages of complement activation. Whereas C3 is required for platelet activation and platelet deposition in vivo, C5 deficiency causes no apparent defect in platelet activation, but nevertheless markedly attenuates TF- and myeloid cell-dependent fibrin formation in venous thrombosis.
Materials and methods
Materials and mice
Sources for antibodies and reagents are described in detail in the supplemental Materials and methods, available on the Blood Web site. C3-deficient B6, 129S4-C3tm1Crr/J and C57BL/6J controls, and C5-deficient B10.D2-Hc0 H2d H2-T18c/oSnJ and strain-matched B10.D2-Hc1 H2d H2-T18c/nSnJ mice were from The Jackson Laboratory (Bar Harbor, ME). Peptidyl-arginine deiminase 4 (PAD4)–deficient mice on a C57BL/6J background were provided by Kerri A. Mowen,32 at The Scripps Research Institute. The generation of TFfl/fl and LysM-Cre+ mice has been previously described.33,34 All procedures were approved by the local committee on legislation on protection of animals (23177-07/G14-1-043; Landesuntersuchungsamt Rheinland-Pfalz, Koblenz, Germany).
Tail-bleeding assay and inferior vena cava (IVC) flow restriction thrombosis model
Tail-bleeding time was assessed as described35 on 6- to 7-week-old male mice under anesthesia. For imaging platelets in vivo, platelets isolated from donor mice were labeled with rhodamine B as reported earlier36 and 250 μL of a 150 × 103 platelets/μL suspension was infused through a jugular vein catheter. The IVC flow restriction thrombosis model was performed as described.37 For partial stenosis, the IVC was ligated over a transiently positioned spacer with a diameter of 0.26 mm.38 This procedure decreases the vascular lumen area to 10% to 15% of the intact vessel and allows for standardized flow restriction without endothelial injury. For assessing thrombosis, the peritoneum and skin were closed, and mice were euthanized after 48 hours for measuring thrombus weight and length.
For dual-visualization of labeled platelets and leukocytes, rhodamine B-labeled platelets and 100 μL of 50 μg/μL acridine orange to label leukocytes were infused through a jugular vein catheter. For dual-visualization of platelets and fibrin, rhodamine B-labeled platelets were injected with Alexa-488 labeled anti-fibrin antibody (2 mg/kg) via the retro-orbital plexus39 in anesthetized mice. To measure PS exposure, mice were subjected to IVC flow restriction for 3 hours received through a jugular vein catheter Alexa Fluor 647 conjugated Annexin V (20 μg per mouse) and fluorescein isothiocyanate anti-mouse Gr-1+ (6 μL per mouse). In some experiments, inhibitors were administered intraperitoneally 15 minutes before surgery. Data were acquired in 1-hour intervals for up to 6 hours in anesthetized mice. Mice that showed more than 5 attached cells in any of the visual fields before ligation or bleeding were excluded.
Four windows were continuously imaged 5 mm away from the ligation site. Measurements were performed with a high-speed wide-field Olympus BX51WI fluorescence microscope using a long-distance condenser and a ×10 (NA 0.3) water immersion objective with a monochromator (MT 20E; Olympus Deutschland GmbH, Hamburg, Germany) and a charge-coupled device camera (ORCA-R2; Hamamatsu Photonics, Shizuoka, Japan).40 For image acquisition and analysis, the Real-time Imaging System eXcellence RT (Olympus Deutschland GmbH, Hamburg, Germany) software was used. Cell recruitment was quantified in 4 fields of view (100 × 150 μm). Transient cells were defined as cells that adhered for 5 to 6 frames but detached. Adherent cells were defined as cells that did not detach from the endothelial lining for 100 frames. Platelets and leukocytes were quantified as cells/mm2 with the real-time Imaging System eXcellence RT (Olympus Deutschland GmbH) software, and fibrin was quantified as percentage of covered area of total visual fields with the Fiji-ImageJ software. For quantification of PS exposure on Gr-1+ myeloid cells, fluorescence intensity was measured on 3 fields per mouse and images were processed with Fiji-ImageJ for determination of integrated intensity with a fixed threshold. The contrast was adjusted to minimize background and autofluorescence between experiments. An appropriate brightness threshold was set for controls and applied to all images within a given experiment for calculation of relative fluorescence intensity of the respective wild-type (WT) controls.41,42
In vitro monocyte experiments
Monocyte TF expression and activity was studied in citrate-anticoagulated whole blood from healthy volunteers stimulated for 4 hours with lipopolysaccharide (LPS) in the presence of the indicated inhibitors. Cells and plasma were separated by double centrifugation. TF procoagulant activity (PCA) in re-calcified platelet-free plasma was measured by single-stage clotting assay calibrated with dilutions of relipidated TF (Innovin; Siemens Healthcare, Erlangen, Germany) in platelet-free pre-stimulation plasma of the same donor. CD14+ monocyte TF antigen expression was determined by flow cytometry.
In vitro platelet studies
Anticoagulated whole blood was obtained by retro-orbital vein or vena cava inferior puncture followed by dilution with 1 volume of Tyrode buffer for preparation of platelet-rich plasma (PRP). Platelets in diluted PRP were stimulated with convulxin with or without PACMA31 (25 μM) as described,43 and stained for activated αIIbβ3 integrin, P-selectin, von Willebrand factor (VWF), or PS for flow cytometry on a FACSCanto II flow cytometer (BD Biosciences). Thrombin generation was assessed in PRP without additional trigger (basal), triggered by TF (1 pM TF, PRP-reagent), or thrombin (0.1 U/mL) with fluorogenic calibrated automated thrombography as described.22,44
Statistics
The software GraphPad Prism (version 5.01 and 6) was used for statistical analysis. For parametric comparison, 2-way analysis of variance (ANOVA), followed by Bonferroni posttests for multiple groups with time-scale observations or 2-tailed Student t test for 2 groups were used. For nonparametric comparison, Wilcoxon-Mann-Whitney U test or Welch’s t test were used. Dose response data were analyzed by 2-way ANOVA followed by Tukey’s multiple comparisons test.
Results
Impaired hemostasis and thrombus formation in C3- and C5-deficient mice
We began characterizing connections between complement and the hemostatic system by evaluating tail-bleeding times in C3- and C5-deficient mice. C3−/− mice showed prolonged time to first cessation of bleeding (supplemental Figure 1A) and total bleeding time (supplemental Figure 1B) compared with co-housed C57BL/6J WT controls, as reported previously.15 Importantly, C5−/− mice showed no difference in the time to first cessation of bleeding (supplemental Figure 1D), but significantly longer total bleeding times (supplemental Figure 1E) relative to strain-matched controls. Because re-bleeding rates were significantly different in C5−/− (supplemental Figure 1F) but not in C3−/− mice (supplemental Figure 1C), these data suggested that C3−/− mice have defective primary hemostasis, whereas C5−/− mice have reduced thrombus stability. Baseline hematologic parameters, including platelet counts, showed no significant difference in C3−/− and C5−/− mice compared with controls (Table 1).
Hematologic parameters of C3−/− and C5−/− mice
| Parameters . | C3+/+ . | C3 −/− . | C5+/+ . | C5−/− . |
|---|---|---|---|---|
| WBC, ×103/μL | 5.58 ± 1.83 | 6.25 ± 2.68 | 9.82 ± 2.5 | 8.22 ± 1.4 |
| RBC, ×103/μL | 8.64 ± 0.119 | 9.20 ± 0.34 | 11.96 ± 2.82 | 8.84 ± 2.02 |
| Hb, g/dL | 13.48 ± 0.17 | 13.87 ± 0.31 | 16.0 ± 2.17 | 13.65 ± 0.23 |
| HCT, % | 45.41 ± 0.57 | 47.31 ± 1.85 | 56.2 ± 9.22 | 50.79 ± 6.74 |
| MCV, fL | 52.46 ± 0.77 | 51.41 ± 0.63 | 55.9 ± 4.12 | 55.21 ± 4.93 |
| MCH, pg | 15.58 ± 0.30 | 15.07 ± 0.29 | 15.97 ± 0.99 | 15.93 ± 1.03 |
| MCHC, g/dL | 29.7 ± 0.67 | 29.32 ± 0.63 | 29.94 ± 2.01 | 30.47 ± 1.71 |
| PLT, ×103/μL | 1152.66 ± 87.80 | 1307 ± 163.50 | 1186.25 ± 142.02 | 1337.66 ± 164.88 |
| Parameters . | C3+/+ . | C3 −/− . | C5+/+ . | C5−/− . |
|---|---|---|---|---|
| WBC, ×103/μL | 5.58 ± 1.83 | 6.25 ± 2.68 | 9.82 ± 2.5 | 8.22 ± 1.4 |
| RBC, ×103/μL | 8.64 ± 0.119 | 9.20 ± 0.34 | 11.96 ± 2.82 | 8.84 ± 2.02 |
| Hb, g/dL | 13.48 ± 0.17 | 13.87 ± 0.31 | 16.0 ± 2.17 | 13.65 ± 0.23 |
| HCT, % | 45.41 ± 0.57 | 47.31 ± 1.85 | 56.2 ± 9.22 | 50.79 ± 6.74 |
| MCV, fL | 52.46 ± 0.77 | 51.41 ± 0.63 | 55.9 ± 4.12 | 55.21 ± 4.93 |
| MCH, pg | 15.58 ± 0.30 | 15.07 ± 0.29 | 15.97 ± 0.99 | 15.93 ± 1.03 |
| MCHC, g/dL | 29.7 ± 0.67 | 29.32 ± 0.63 | 29.94 ± 2.01 | 30.47 ± 1.71 |
| PLT, ×103/μL | 1152.66 ± 87.80 | 1307 ± 163.50 | 1186.25 ± 142.02 | 1337.66 ± 164.88 |
Hb, hemoglobin; HCT, hematocrit; MCH, mean corpuscular Hb; MCHC, mean corpuscular Hb concentration; MCV, mean corpuscular volume; PLT, platelet; RBC, red blood cell; WBC, white blood cell.
We used the IVC thrombosis model to analyze the role of the complement system in venous thrombosis. The IVC was ligated to induce a flow restriction of ∼85% to 90%, which initiates thrombus formation resembling the histology of human venous thrombi.45 Significantly fewer C3−/− mice had visible thrombi 48 hours after induction of flow restriction (<30% of C3−/− mice vs ∼80% of WT controls). In addition, weight and length (Figure 1A-B) of thrombi were lower in C3−/− mice as compared with WT controls, in accord with prior data in photochemical injury of arterioles and venules of C3−/− mice.15 C5−/− mice also had reduced thrombus length and weight compared with WT controls (Figure 1C-D), despite similar incidence of visible thrombi (>90%). Thus, complement activation contributed to thrombus formation in the IVC flow-restriction venous thrombosis model.
Impaired thrombus formation in C3- and C5-deficient mice. C3−/− mice have less thrombus weight (A) and length (B) compared with C3+/+ controls 48 hours after IVC stenosis (n = 9-11; mean ± standard deviation [SD]; Mann-Whitney U test). *P < .05; **P < .01. C5−/− mice have less thrombus weight (C) and length (D) compared with C5+/+ controls 48 hours after IVC stenosis (n = 11-12; mean ± SD; Mann-Whitney U test). Adherent (E) and rolling (F) leukocytes, and adherent (G) and transient (H) platelets following IVC low restriction were compared between C3−/− mice and WT controls by intravital imaging over 6 hours (n = 6; mean ± SD); 2-way ANOVA for multiple groups with time scale observations followed by Bonferroni posttest; *P < .05, **P < .01, ***P < .001. h, hour; n.s., not significant. Red circles represent WT; blue squares represent knock-out.
Impaired thrombus formation in C3- and C5-deficient mice. C3−/− mice have less thrombus weight (A) and length (B) compared with C3+/+ controls 48 hours after IVC stenosis (n = 9-11; mean ± standard deviation [SD]; Mann-Whitney U test). *P < .05; **P < .01. C5−/− mice have less thrombus weight (C) and length (D) compared with C5+/+ controls 48 hours after IVC stenosis (n = 11-12; mean ± SD; Mann-Whitney U test). Adherent (E) and rolling (F) leukocytes, and adherent (G) and transient (H) platelets following IVC low restriction were compared between C3−/− mice and WT controls by intravital imaging over 6 hours (n = 6; mean ± SD); 2-way ANOVA for multiple groups with time scale observations followed by Bonferroni posttest; *P < .05, **P < .01, ***P < .001. h, hour; n.s., not significant. Red circles represent WT; blue squares represent knock-out.
Platelet, but not leukocyte deposition is reduced in C3−/− mice following IVC stenosis
We characterized details of thrombus formation in the IVC by wide-field intravital epifluorescent microscopy. Because venous thrombosis is dependent on both leukocytes and platelets,46 we followed the interactions of blood cells with the vessel wall. Typical images for the deposition of acridine orange-labeled leukocytes and rhodamine B-labeled platelets after flow restriction (0, 3, and 6 hours) are shown in supplemental Videos 1 and 2.
We first quantified leukocyte rolling and firm attachment over an extended time period following ligation-induced flow restriction of the IVC. Adhesion of leukocytes was comparable during the initial 4 hours of thrombus formation in flow-restricted vessels of C3−/− and WT controls (Figure 1E-F). In contrast, quantifications of transient interactions of platelets and of firm adhesion of platelets with the vessel wall were markedly reduced as early as 2 hours after ligation of the IVC in C3−/− vs WT mice (Figure 1G-H). These data demonstrated that platelet deposition was dependent on complement activation in the IVC stenosis model of venous thrombosis.
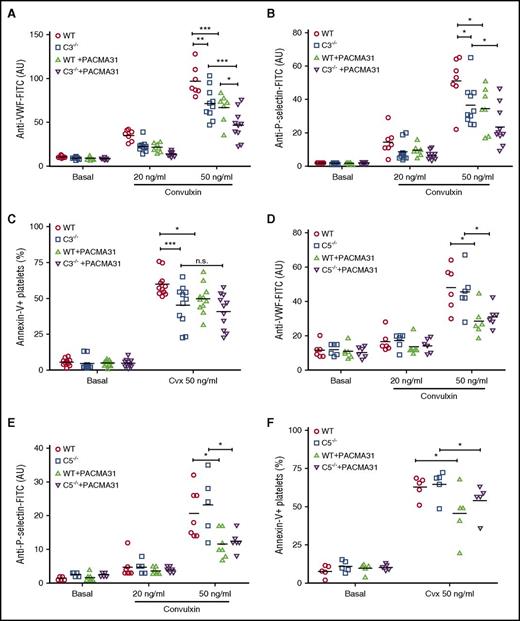
We therefore studied platelet function in vitro. Because platelets may be influenced by deposition of the lytic terminal C5b-9 complex,12 we compared the activation of platelets from C3- and C5-deficient mice. Activation of platelets with the collagen receptor GPVI agonist convulxin in C3-deficient plasma resulted in diminished VWF binding (Figure 2A), P-selectin exposure (Figure 2B), and integrin αIIbβ3 activation (supplemental Figure 2A). Exposure of procoagulant PS was also modestly diminished on convulxin-stimulated platelets in C3-deficient plasma (Figure 2C), but TF, thrombin, or convulxin induced similar thrombin production in PRP from C3−/− vs WT mice (supplemental Figure 2B). Taken together, these data indicated that complement only minimally increases platelet procoagulant properties. Although the effects of C3 deficiency on platelet activation were consistent with prior reports implicating complement interaction with platelets,47,48 activation of platelets in C5-deficient plasma had no effect on VWF binding (Figure 2D), P-selectin exposure (Figure 2E), integrin αIIbβ3 activation (supplemental Figure 2C), the exposure of procoagulant PS (Figure 2F), or platelet-dependent thrombin generation (supplemental Figure 2D). Thus, complement activation influences platelets essentially independent of the terminal C5b-9 complement complex.
Platelet activation depends on C3 but not on C5. Flow cytometry detection of VWF (A), P-selectin (B), and PS exposure (C) on convulxin (Cvx)–stimulated platelets in PRP from C3−/− mice and respective WT controls in the absence or presence of PACMA31 (25 μM) (n = 6-11). Similarly, flow cytometry detection of VWF (D), P-selectin (E), and PS exposure (F) on Cvx-stimulated platelets in PRP from C5−/− mice and respective WT controls in the absence or presence of PACMA 31 (25 μM) (n = 5-11). VWF and P-selection surface expression are shown as linear arbitrary units (AU). PS exposure data are shown as percentage of annexin V+ platelets. Data are presented as mean ± SD and were analyzed by 2-way ANOVA followed by Tukey's multiple comparisons test. *P < .05; **P < .01; ***P < .001. FITC, fluorescein isothiocyanate; n.s., not significant.
Platelet activation depends on C3 but not on C5. Flow cytometry detection of VWF (A), P-selectin (B), and PS exposure (C) on convulxin (Cvx)–stimulated platelets in PRP from C3−/− mice and respective WT controls in the absence or presence of PACMA31 (25 μM) (n = 6-11). Similarly, flow cytometry detection of VWF (D), P-selectin (E), and PS exposure (F) on Cvx-stimulated platelets in PRP from C5−/− mice and respective WT controls in the absence or presence of PACMA 31 (25 μM) (n = 5-11). VWF and P-selection surface expression are shown as linear arbitrary units (AU). PS exposure data are shown as percentage of annexin V+ platelets. Data are presented as mean ± SD and were analyzed by 2-way ANOVA followed by Tukey's multiple comparisons test. *P < .05; **P < .01; ***P < .001. FITC, fluorescein isothiocyanate; n.s., not significant.
C5 specifically contributes to fibrin formation in venous thrombosis
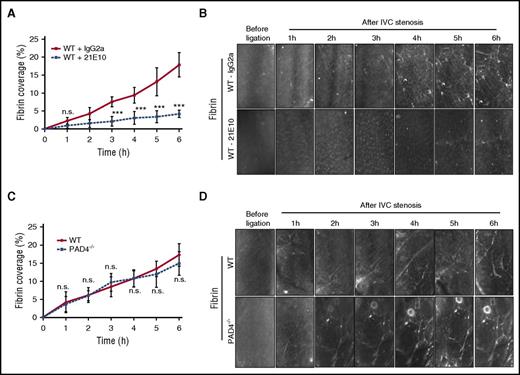
In another series of experiments, we simultaneously quantified platelet and fibrin deposition. Adherent (Figure 3A) and transient platelets (Figure 3B) were reduced, confirming the reproducibility of the defective platelet function in C3−/− mice. C3−/− mice had also significantly reduced fibrin formation under IVC flow restriction (Figure 3C-D). In line with prior studies linking P-selectin and C3 activation,12 blocking the P-selectin ligand PSGL-1 similarly reduced platelet and fibrin deposition (supplemental Figure 3). Because platelet activation47,49 and the platelet ligand VWF are regulated by thiol-disulfide exchange reactions50-52 and C5 conversion, but not further downstream complement components, and is known to alter the redox state of cell surface PDI,18,53 we compared the inhibitory effects of C3 deficiency with inhibition of PDI by PACMA31. PACMA31 has an optimal fit with the second catalytic domain of PDI,54,55 and this PDI domain has recently been shown to be crucial for regulating platelet activation and thrombosis in vivo.31,56 Under the experimental conditions, addition of the PDI inhibitor PACMA31 produced significant and similar inhibition of these platelet activation parameters in WT and C5-deficient plasma (Figure 2D-F and supplemental Figure 2C), indicating that C5 activation is not connected to PDI roles in platelet activation. Importantly, PACMA31 added to C3−/− plasma also further suppressed convulxin-induced VWF binding, integrin αIIbβ3 activation, and P-selectin exposure, but showed no significant additive inhibition of the reduced platelet surface PS exposure seen in C3−/− mice (Figure 2A-C and supplemental Figure 2A). These data indicated that C3 activation has partially overlapping, but also distinct roles in thiol-disulfide exchange and PDI-dependent platelet responses.
Reduced fibrin formation in C3−/− mice in venous thrombosis. Adherent (A) and transient (B) platelets, and fibrin coverage (C) were compared between C3−/− mice and WT controls under IVC stenosis condition and imaged for over 6 hours using intravital microscopy (D) (n = 4-5; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttest. *P < .05; **P < .01; ***P < .001. h, hour; n.s., not significant.
Reduced fibrin formation in C3−/− mice in venous thrombosis. Adherent (A) and transient (B) platelets, and fibrin coverage (C) were compared between C3−/− mice and WT controls under IVC stenosis condition and imaged for over 6 hours using intravital microscopy (D) (n = 4-5; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttest. *P < .05; **P < .01; ***P < .001. h, hour; n.s., not significant.
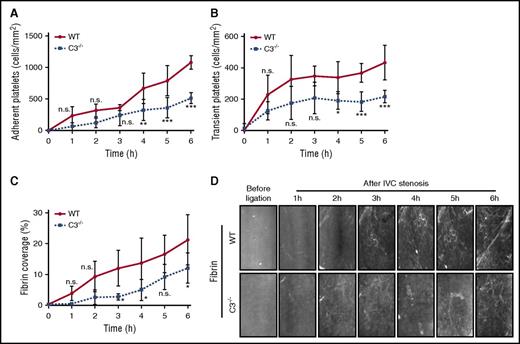
Consistent with the presented in vitro data, transient and firm platelet adhesion to the flow-restricted IVC was reduced in mice treated with the PDI inhibitor PACMA31 (Figure 4A-B), and local fibrin formation was markedly impaired relative to untreated controls (Figure 4C-D). In contrast, transient and firm platelet interactions with the ligated IVC were indistinguishable between C5−/− and WT control mice (Figure 4E-F). Remarkably, despite normal platelet deposition, fibrin formation was reduced in C5-deficient mice (Figure 4G-H) to a similar extent as seen in mice treated with the PDI inhibitor (Figure 4C-D). Because platelet activation in vitro, including the exposure of procoagulant PS, was unchanged in C5−/− plasma, these data indicated an unexpected role for C5 in activating prothrombotic responses involving vessel wall or blood cells other than platelets.
C5 specifically contributes to fibrin formation in venous thrombosis. Effect of the PDI inhibitor PACMA31 on adherent (A) and transient (B) platelets, and fibrin formation (C) under an IVC stenosis condition. Mice were treated 30 minutes before surgery and imaged over 6 hours using intravital microscopy (D) (n = 3; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were compared (treatment vs dimethyl sulfoxide [DMSO] controls) by 2-way ANOVA followed by Bonferroni posttests. P < .05. Adherent (E) and transient (F) platelets, and fibrin formation (G) were compared between C5−/− mice and WT controls under an IVC stenosis condition and imaged over 6 hours using intravital microscopy (H) (n = 6; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttest. *P < .05; **P < .01; ***P < .001. h, hour; n.s., not significant.
C5 specifically contributes to fibrin formation in venous thrombosis. Effect of the PDI inhibitor PACMA31 on adherent (A) and transient (B) platelets, and fibrin formation (C) under an IVC stenosis condition. Mice were treated 30 minutes before surgery and imaged over 6 hours using intravital microscopy (D) (n = 3; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were compared (treatment vs dimethyl sulfoxide [DMSO] controls) by 2-way ANOVA followed by Bonferroni posttests. P < .05. Adherent (E) and transient (F) platelets, and fibrin formation (G) were compared between C5−/− mice and WT controls under an IVC stenosis condition and imaged over 6 hours using intravital microscopy (H) (n = 6; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttest. *P < .05; **P < .01; ***P < .001. h, hour; n.s., not significant.
Fibrin formation is dependent on TF expression by myeloid cells
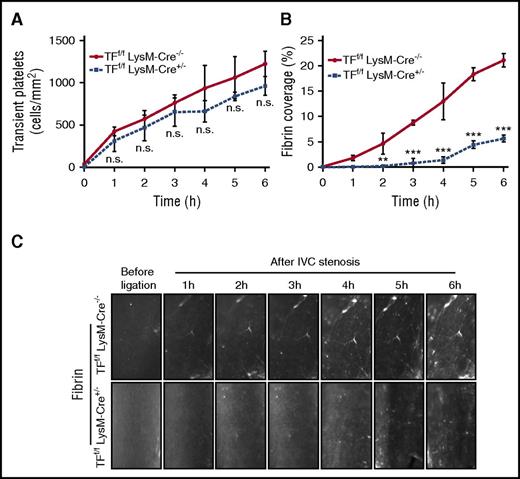
We have previously shown that binding of the complement fixing anti-thymocyte globulin, induces TF activation on isolated cultured monocytes dependent on thiol-disulfide exchange involving PDI and C5.18 In addition, TF activation required exposure of cell surface PS caused by C5b-C7 membrane insertion, but not the fully assembled C5b-C9 membrane attack complex. Given the complement regulation of blood monocyte TF activity, we analyzed whether TF expressed by myeloid cells was required for fibrin formation following flow restriction of the IVC. Myeloid cell TF deficiency in TFf/f LysM-Cre+/− mice had no effect on transient platelet interactions with the vessel wall (Figure 5A), but at later times produced significantly reduced firm adhesion of platelets to the flow-restricted IVC (supplemental Figure 4A). Importantly, TFf/f LysM-Cre+/− mice showed a markedly reduced fibrin formation relative to Cre−/− littermate controls (Figure 5B-C), demonstrating that coagulation activation under IVC stenosis condition in vivo is myeloid cell TF dependent.
Fibrin formation is dependent on TF expression by myeloid cells. Transient platelets (A) and fibrin formation (B) were compared between myeloid cell-specific TF-deficient mice (TFf/f LysM-Cre+/−) with controls (TFf/f LysM-Cre−/−) following IVC stenosis over 6 hours (C) (n = 3; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttest. **P < .01; ***P < .001. h, hour; n.s., not significant.
Fibrin formation is dependent on TF expression by myeloid cells. Transient platelets (A) and fibrin formation (B) were compared between myeloid cell-specific TF-deficient mice (TFf/f LysM-Cre+/−) with controls (TFf/f LysM-Cre−/−) following IVC stenosis over 6 hours (C) (n = 3; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttest. **P < .01; ***P < .001. h, hour; n.s., not significant.
We confirmed the TF dependence of fibrin formation using a function blocking anti-TF antibody.21 We injected TF antibody 21E10 or isotype-matched rat immunoglobulin G2a (IgG2a) control 30 minutes before IVC stenosis, and imaged fibrin formation over 6 hours in C57BL/6J mice. Mice receiving the specific anti-TF antibody also showed a marked reduction in fibrin formation compared with IgG2a control-treated mice (Figure 6A-B). TF blockade had no effect on transient platelet interactions with the vessel wall (supplemental Figure 4B), and similar to TF deletion in myeloid cells, caused moderately reduced platelet firm adhesion (supplemental Figure 4C). Thus, pharmacologic and genetic evidence showed that TF plays a major role in fibrin formation in the flow-restriction IVC thrombosis model.
Fibrin formation is dependent on TF and independent of NET formation. (A) Fibrin staining in TF antibody (21E10) or isotype control (IgG2a)-treated mice following IVC stenosis over 6 hours. (B) Quantification of fibrin deposition (n = 5-6; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttests. ***P < .001. (C) Fibrin formation in the stenosed IVC of PAD4−/− mice and WT mice over 6 hours. (D) Quantification of fibrin deposition. Scale bar: 100 μm (n = 5-6; 3-4 visual fields per mouse; mean ± SD). Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttests. h, hour; NET, neutrophil extracellular trap; n.s., not significant.
Fibrin formation is dependent on TF and independent of NET formation. (A) Fibrin staining in TF antibody (21E10) or isotype control (IgG2a)-treated mice following IVC stenosis over 6 hours. (B) Quantification of fibrin deposition (n = 5-6; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttests. ***P < .001. (C) Fibrin formation in the stenosed IVC of PAD4−/− mice and WT mice over 6 hours. (D) Quantification of fibrin deposition. Scale bar: 100 μm (n = 5-6; 3-4 visual fields per mouse; mean ± SD). Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttests. h, hour; NET, neutrophil extracellular trap; n.s., not significant.
NETs resulting from the release of decondensed chromatin were found to be part of the thrombus scaffold in venous thrombosis models.45 In the absence of defects in early platelet deposition, venous thrombi generated by permanent IVC ligation were smaller in PAD4-deficient mice leading to reduced histone citrullination, a process required for chromatin decondensation and NET formation. TF upregulation in human neutrophils was also implicated in triggering arterial thrombosis.57 We therefore addressed whether PAD4 was required for fibrin formation in the flow-restricted IVC. Control experiments confirmed that neutrophils from PAD4−/− mice did not form citrullinated NETs in vitro (supplemental Figure 5). In contrast to myeloid cell deletion of TF, fibrin formation and platelet adhesion were not different in the flow-restricted IVC of WT vs PAD4−/− mice for the initial 5 hours of the observation period (Figure 6C-D; supplemental Figure 4D). These results indicated that NETosis does not play a major role in coagulation activation and fibrin formation in the early stages of venous thrombus formation.
C5 is required for leukocyte PS exposure in the flow-restricted IVC
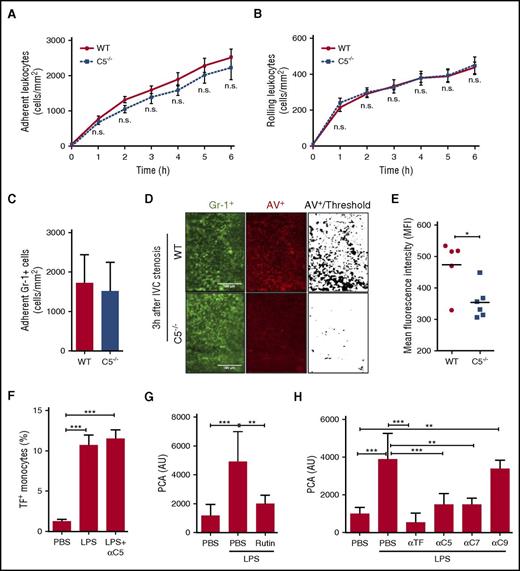
Although C5a is an anaphylatoxin involved in leukocyte recruitment, we observed similar leukocyte adhesion (Figure 7A) and rolling (Figure 7B) in C5−/− mice and WT strain-matched controls. Similarly, quantification of GR-1+ myeloid cells showed no apparent difference in leukocyte recruitment at the vessel wall 3 hours after induction of flow restriction (Figure 7C). Given the suggested role for complement C5 in monocyte TF activation dependent on negatively charged phospholipid, we next quantified PS exposure on leukocytes in C5−/− mice and WT strain-matched controls (Figure 7D). Annexin V-staining to detect PS+ cells among the leukocyte population showed significantly less fluorescence intensity in C5−/− mice relative to WT controls (Figure 7E). These data showed that C5 plays a significant role in regulating leukocyte procoagulant phospholipid exposure and fibrin formation in venous thrombosis.
C5 is required for leukocyte PS exposure in the flow-restricted IVC. Adhesion (A) and rolling (B) of leukocytes were investigated in C5−/− mice and WT controls under IVC stenosis condition over 6 hours (n = 5-6; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttests. (C) Quantification of adherent Gr-1+ cells 3 hours after IVC ligation. (D) PS exposure was measured in C5−/− mice and WT controls 3 hours after flow restriction of the IVC. Fluorescence labeled Gr-1+ to stain myeloid cells (green) and Annexin V (AV) to identify PS+ cells (red) were injected prior to imaging. (E) Quantification of AV staining in C5−/− mice and WT controls. Two to 3 visual fields per mouse were analyzed with fixed threshold, and relative fluorescence of WT is shown as mean ± SD and analyzed by Mann-Whitney U test. *P < .05. (F) Citrated whole blood was stimulated with LPS for 4 hours in the presence and absence of anti-C5 antibody, and TF cell-surface expression was measured on CD14+ monocytes. Data were expressed as mean ± SD; n = 3. Data were analyzed by 1-way ANOVA followed by Bonferroni posttests. ***P < .001. (G-H) Citrated whole blood was stimulated with LPS in the presence of 100 μM rutin (G), anti-C5, C7, or C9 antibody (H) for 4 hours, and the PCA was measured in cell-free plasma. Anti-TF was added to the clotting assay to demonstrate induction of TF activity in plasma from LPS-stimulated blood. Data were expressed as mean ± SD; n = 3 to 6. Data were analyzed by 1-way ANOVA followed by Bonferroni posttests. **P < .01; ***P < .001. MFI, mean fluorescence intensity; n.s., not significant; PBS, phosphate-buffered saline.
C5 is required for leukocyte PS exposure in the flow-restricted IVC. Adhesion (A) and rolling (B) of leukocytes were investigated in C5−/− mice and WT controls under IVC stenosis condition over 6 hours (n = 5-6; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttests. (C) Quantification of adherent Gr-1+ cells 3 hours after IVC ligation. (D) PS exposure was measured in C5−/− mice and WT controls 3 hours after flow restriction of the IVC. Fluorescence labeled Gr-1+ to stain myeloid cells (green) and Annexin V (AV) to identify PS+ cells (red) were injected prior to imaging. (E) Quantification of AV staining in C5−/− mice and WT controls. Two to 3 visual fields per mouse were analyzed with fixed threshold, and relative fluorescence of WT is shown as mean ± SD and analyzed by Mann-Whitney U test. *P < .05. (F) Citrated whole blood was stimulated with LPS for 4 hours in the presence and absence of anti-C5 antibody, and TF cell-surface expression was measured on CD14+ monocytes. Data were expressed as mean ± SD; n = 3. Data were analyzed by 1-way ANOVA followed by Bonferroni posttests. ***P < .001. (G-H) Citrated whole blood was stimulated with LPS in the presence of 100 μM rutin (G), anti-C5, C7, or C9 antibody (H) for 4 hours, and the PCA was measured in cell-free plasma. Anti-TF was added to the clotting assay to demonstrate induction of TF activity in plasma from LPS-stimulated blood. Data were expressed as mean ± SD; n = 3 to 6. Data were analyzed by 1-way ANOVA followed by Bonferroni posttests. **P < .01; ***P < .001. MFI, mean fluorescence intensity; n.s., not significant; PBS, phosphate-buffered saline.
Because platelets supported complement activation in the IVC thrombosis model, we reasoned that other perturbations in blood might also link complement to TF activation. We mimicked septic conditions by stimulating whole blood anticoagulated with citrate in the presence of LPS for 4 hours in order to induce TF expression in monocytes. Blocking the complement component C5 did not inhibit TF cell surface expression detected by flow cytometry (Figure 7F), excluding that C5a receptors were required for TF induction. Measurements of TF activity in platelet-free plasma showed that plasma TF activity was associated with released microvesicles. Importantly, blocking PDI with rutin (Figure 7G),18 C5 or C7 but not C9 (Figure 7H), markedly reduced TF PCA in the plasma isolated after LPS stimulation. Thus, TF antigen was upregulated on the surface of monocytes independent of C5, but C5 and C7 activation played a crucial role in generating procoagulant TF in whole blood.
Discussion
In this study, we show a link between the hemostatic and complement systems in venous thrombosis. Although prior studies indicated that complement influences platelet activation,58 our data show that platelet interaction with the flow-restricted venous vessel wall is largely independent of formation of the terminal complement complex. Rather, C5-dependent membrane perturbations specifically lead to prothrombotic TF activation on myeloid cells and thus contribute to fibrin formation in thrombosis, whereas C3 activation contributes to platelet activation as well as fibrin formation.
Our data indicate that C3−/− mice are impaired in primary hemostasis and C5−/− mice have reduced thrombus stability following injury. Both mice displayed reduced thrombus burden 48 hours after flow restriction of the IVC. These findings are in line with prior studies implicating complement in thrombosis. Deletions of the complement lectin pathway components, mannose-binding lectin and mannose-binding lectin-associated serine protease-1/-3 but not C2/factor B, prevent ferric chloride-induced arterial thrombosis,59 and triggering platelets leads to activation of mannose-binding lectin-associated serine protease-1 and -3.60 Hemostasis and thrombus formation in a photochemical thrombosis model were also impaired in C3−/− mice.15 Activation of C3, but not the alternative complement pathway component C4, on platelets is important for opsonizing bacteria for glycosylphosphatidylinositol b-dependent uptake by CD8+ cross-presenting dendritic cells.61 Consistent with these demonstrated roles of C3 interacting with platelets, we found that convulxin activation of platelets from C3−/− mice resulted in reduced VWF binding, P-selectin exposure, integrin αIIbβ3 activation, and exposure of procoagulant PS. In contrast, these platelet activation markers were unchanged upon stimulation of PRP from C5−/− mice.
It is of interest that platelets are endowed with a variety of mechanisms to regulate in situ complement activation. Platelets contain C3,62 and C1 inhibitor which is stored in α-granules and associates upon platelet activation with P-selectin,63 thereby interfering with leukocyte rolling on endothelial cells.63,64 Platelets can release factor H that binds to platelet integrins65 and express surface membrane-anchored complement regulatory proteins such as CD55, CD59, and clusterin.66 These mechanisms indicate functional redundancy to prevent excessive complement activation on platelets during hemostasis and physiological host defense. Control of complement activation may be particularly important following endothelial activation during inflammation. Complement on the one hand can induce VWF release from endothelial cells,67 and VWF in turn has a complex role in coagulation68 and reciprocally regulates complement.69 Specifically, ultra-large VWF multimers, as observed after tissue injury, can provide a binding platform for C3b to trigger complement activation.1,70,71
Although direct reciprocal activation of complement and coagulation was indicated by several studies,7 complement activation is also known to alter cell surface activity of PDI that is increasingly recognized for its role in vascular thrombosis.29 Platelet activation and VWF are regulated by thiol-disulfide exchange reactions49-51 and C3-dependent activation of C5 is known to modify the redox state of cell surface PDI.18,53 Inhibition of PDI had additive effects with C3 deficiency on reducing platelet activation with the exception of a common modest reduction in platelet PCA by both, consistent with a minor role of complement in contributing to PDI-dependent platelet thiol-disulfide exchange reactions. However, we found that the PDI inhibitor, PACMA31, inhibited not only IVC flow-restriction–induced platelet deposition, but also fibrin formation that was dependent on myeloid cell-expressed TF. Importantly, C5-deficient mice had no apparent defect in platelet or leukocyte interactions at the flow-restricted vessel wall, but were defective in fibrin deposition to a similar extent as seen in PDI-inhibitor–treated mice. Although the precise biochemical reaction controlled by PDI cannot be defined by in vivo studies, these data suggest a link between complement C5 activation, PDI, and TF prothrombotic activity.
The effects of C5 deficiency are consistent with our previous demonstration that complement activation triggers monocyte TF activation by C5-dependent thiol-disulfide exchange and initial membrane insertion of downstream complement components. Accordingly, we here show that the exposure of PS on adherent leukocytes in the flow-restricted vessel wall was reduced in C5-deficient mice as compared with WT mice. In addition, we provide evidence that the inflammatory induction of TF antigen in whole blood monocytes by LPS was not dependent on complement, in sharp contrast to the release of TF activity that was markedly reduced by the PDI inhibitor Rutin or antibodies to C5 and C7, but not C9. These data demonstrating that TF activation in monocytes is under control of the complement cascade, both in venous thrombosis and inflammation, expand previous studies by implicating leukocytes in coagulation-complement crosstalks.
Although these experiments delineate platelet and coagulation activation mechanisms in early thrombus development, venous thrombosis and its resolution will be determinant by additional factors in clinical settings. The presented data, however, shed new light on the high prevalence of thrombo-embolic complications in paroxysmal nocturnal hemoglobinuria.72 Lack of glycosylphosphatidylinositol-anchored complement regulatory proteins (CD55 and CD59) have already been linked to enhanced generation of prothrombotic microparticles73 and reduced regulation of TF by the TF pathway inhibitor.74,75 The demonstrated contribution of the complement cascade to activation of monocyte-expressed TF may amplify this prothrombotic state and precipitate serious thrombosis, when TF levels are elevated in the context of chronic inflammation or acute infection. Given the success of complement inhibition in this disease,4 one may expect benefits from intervening into TF activation through complement blockade or PDI inhibitors in other disorders of thrombo-inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study was supported by grants from the Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503) (S.S., K.J., L.H., S.J., C.R., P.W., and W.R.), the Alexander von Humboldt Foundation (W.R.), and the National Institutes of Health National Heart, Lung, and Blood Institute (HL31950 and UM1 HL120877) (W.R.).
Authorship
Contribution: S.S. performed and analyzed in vivo intravital microscopy experiments; K.J., K.S., L.H., S.J., F.L., M.S., C.R., and P.W. designed, performed, and analyzed experiments; and S.S. and W.R. designed the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfram Ruf, Center for Thrombosis and Hemostasis, University Medical Center of the Johannes Gutenberg University, Langenbeckstr 1, D-55131 Mainz, Germany; e-mail: ruf@uni-mainz.de.

![Figure 1. Impaired thrombus formation in C3- and C5-deficient mice. C3−/− mice have less thrombus weight (A) and length (B) compared with C3+/+ controls 48 hours after IVC stenosis (n = 9-11; mean ± standard deviation [SD]; Mann-Whitney U test). *P < .05; **P < .01. C5−/− mice have less thrombus weight (C) and length (D) compared with C5+/+ controls 48 hours after IVC stenosis (n = 11-12; mean ± SD; Mann-Whitney U test). Adherent (E) and rolling (F) leukocytes, and adherent (G) and transient (H) platelets following IVC low restriction were compared between C3−/− mice and WT controls by intravital imaging over 6 hours (n = 6; mean ± SD); 2-way ANOVA for multiple groups with time scale observations followed by Bonferroni posttest; *P < .05, **P < .01, ***P < .001. h, hour; n.s., not significant. Red circles represent WT; blue squares represent knock-out.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/16/10.1182_blood-2016-11-749879/4/m_blood749879f1.jpeg?Expires=1770912521&Signature=RDONYpFdbF~jz-aOTm9mUltkQ7BPaT-SKc7piRxcXRebpxJtz~bw49Gi0h~dEVQ3xHL3mTTGpXWpqFnpq1Ygc8bREx8lDKHEDzfM0KRlqp6MHRfNTFVuijoAACd2PRzl8WPPh~fWBTr7-F5guy4DrRsHXB17GjZRm13u7irCAjAo4GFLMeKUdbTv9Z6F2-q0JN7do2YlU94zwUdWeJYjj1F1AI6DddZfjYDGtzq3SL7zLrczdr7V70t7bw-kO-G3uj1Z3usPlJTLA3C5hv~G09YqJvWHR6TXUgmWqHR5a9SQZWTCVEgWugKwIK-Jv3KDbW8XCPGqCPfiP4Ksujd2Mg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. C5 specifically contributes to fibrin formation in venous thrombosis. Effect of the PDI inhibitor PACMA31 on adherent (A) and transient (B) platelets, and fibrin formation (C) under an IVC stenosis condition. Mice were treated 30 minutes before surgery and imaged over 6 hours using intravital microscopy (D) (n = 3; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were compared (treatment vs dimethyl sulfoxide [DMSO] controls) by 2-way ANOVA followed by Bonferroni posttests. P < .05. Adherent (E) and transient (F) platelets, and fibrin formation (G) were compared between C5−/− mice and WT controls under an IVC stenosis condition and imaged over 6 hours using intravital microscopy (H) (n = 6; 3-4 visual fields per mouse; mean ± SD). Scale bar: 100 μm. Consecutive measurements were evaluated by 2-way ANOVA followed by Bonferroni posttest. *P < .05; **P < .01; ***P < .001. h, hour; n.s., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/16/10.1182_blood-2016-11-749879/4/m_blood749879f4.jpeg?Expires=1770912521&Signature=EHRlvFYWuLw5OzDCRyRpVsbRJ5PKNZ-rztQ6KOLnfn4aAcyhMZJAp9FgJXMotFpuTABaLc7hU1fs7o7vxhj6HkZvS8bemW-sY7P3tSO1Nzn4S6KdhUi7ET1AoLxiUgFEjSqZec9p-RvegpkO4ZRlgTVW8lrrn1QJhu2OVogL8Gu5KdDxPrv9Mwt19bfUKzGL0MEbEYldP9ENv-48As7ex~De1WAYhdTR0B-ps60pnb9aASGRd2hm1JHreJ-2SXtmChAZAc93paKnLH~xVPq9tJj2UNyTSmLDiOiBscvAKrogIdoeifbat8LSZUaGPzldkv5fZBeBkClJpfKV63wH-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)