Key Points
Thrombophilia in children with perinatal stroke is rare, with rates similar to those in the normal population.
Routine testing in childhood is not indicated.
Abstract
Perinatal stroke causes cerebral palsy and lifelong disability. Specific diseases are definable, but mechanisms are poorly understood. Evidence suggests possible associations between arterial perinatal stroke and prothrombotic disorders, but population-based, controlled, disease-specific studies are limited. Understanding thrombophilia in perinatal stroke informs pathogenesis models and clinical management. We conducted a population-based, prospective, case-control study to determine the association of specific perinatal stroke diseases with known thrombophilias. Children with idiopathic magnetic resonance imaging–classified neonatal arterial ischemic stroke (NAIS), arterial presumed perinatal ischemic stroke (APPIS), or fetal periventricular venous infarction (PVI) were recruited. Standardized thrombophilia evaluations were performed after 12 months of age on stroke cases and controls, including quantified proteins C and S, antithrombin, factors VIII/IX/XI, fibrinogen, lipoprotein(a), homocysteine, lupus anticoagulant, anticardiolipin antibodies and genotyping of factor V Leiden (FVL), factor II G20210A (FII), and methylenetetrahydrofolate reductase C677T. A total of 212 children were studied: 46 with NAIS, 34 with APPIS, 55 with PVI, and 77 controls (male, 53%; median age, 4.8 years). Of 14 parameters, no differences were observed in 12, including all common thrombophilias. Mean prothrombin time was shorter in arterial strokes (P < .001). Rates of antiphospholipid antibodies were low, comparable to those in controls, and resolved on repeat testing. FVL and FII rates were comparable to population norms. Total number of possible abnormalities did not differ between cases and controls. Our prospective, population-based, controlled, disease-specific study suggests minimal association between perinatal stroke and thrombophilia. This does not exclude the possibility of disordered coagulation at the time of stroke but suggests testing in childhood is not indicated.
Medscape Continuing Medical Education online
This activity has been planned and implemented through the joint providership of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the American Nurses Credentialing Center (ANCC), the Accreditation Council for Pharmacy Education (ACPE), and the Accreditation Council for Continuing Medical Education (ACCME), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2820.
Disclosures
Associate Editor Thomas L. Ortel served as an advisor or consultant for Instrumentation Laboratory. CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Alnylam, Biogen, and Pfizer. Author Patricia Massicotte served on the steering committee for Bayer, on the clinical events committee for Xeltis, and as a site investigator for Pfizer and Daiichi Sankyo. The remaining authors declare no competing financial interests.
Learning objectives
Identify relative rates of thrombophilias in children with perinatal stroke based on a prospective, case-control study.
Distinguish findings of genotyping for prothrombotic disorders in children with perinatal stroke.
Determine the clinical implications of this prospective, case-control study, including the need for testing in children with perinatal stroke.
Release date: May 18, 2017; Expiration date: May 18, 2018
Introduction
Perinatal stroke is the leading cause of hemiparetic cerebral palsy and results in lifelong disability.1 Disease-specific subtypes of perinatal stroke are now definable based on clinical presentation and neuroimaging including vascular distribution.2,3 Most common are arterial ischemic strokes, but fetal venous infarcts also occur. There are many proposed risk factors, but pathogenesis remains unclear in most cases.4 Study of these specific disease states is required to elucidate pathophysiological mechanisms if prevention strategies are to be realized.
Neonatal arterial ischemic stroke (NAIS) presents acutely in the first days of life, typically with seizures.3 Diffusion magnetic resonance imaging (MRI) confirms recent arterial infarction. Arterial presumed perinatal ischemic stroke (APPIS) presents later in childhood, typically with motor asymmetry at 4 to 6 months.5,6 MRI shows remote arterial infarction, most often combined cortical and subcortical injuries in the middle cerebral artery territory indistinguishable from the chronic appearance of NAIS. Accordingly, NAIS and APPIS may represent the same disease, differing only in timing of symptomatic presentation. The pathogenesis of such arterial perinatal strokes is poorly understood. Case-control studies are limited,7-10 and a recent meta-analysis11 has methodological issues. Prevailing theory suggests many arterial perinatal strokes are thromboembolic events, possibly arising from the placenta. Most studies of thrombophilia in perinatal stroke have only included NAIS.
Another common perinatal stroke disease is fetal periventricular venous infarction (PVI). PVI also presents later in infancy after term birth, typically with early hand preference and hemiparesis.6 Modern neuroimaging has confirmed that PVI represents an in utero germinal matrix hemorrhage with secondary venous infarction of the periventricular white matter.2,12-14 In the term infant presenting with hemiparesis, PVI is increasingly recognized.12,14 Many risk factors have been identified for germinal matrix hemorrhage in preterm infants, but this fetal correlate has been less studied. The role of the coagulation system in germinal matrix hemorrhage and secondary venous infarction is unclear, regardless of gestational age.15 Prothrombotic abnormalities have been associated with preterm PVI, but only small, uncontrolled studies exist in term PVI.6
The role of prothrombotic abnormalities in perinatal stroke has been investigated previously but almost exclusively in NAIS populations. Studies have been limited by absence of controls, retrospective design, highly selected and modestly powered samples, inconsistent laboratory methods, and heterogeneous populations combining those with less specific cerebral palsy or perinatal stroke without specific, MRI-classified stroke disease states.16 Studies in presumed perinatal stroke (APPIS, PVI) have been even more limited,5,6,17,18 with no disease-specific, well-powered, case-control studies to date. A meta-analysis of thrombophilia in pediatric stroke was unable to examine perinatal populations, let alone each specific perinatal stroke disease in isolation.19
A long list of known prothrombotic conditions have been investigated in perinatal stroke. These are summarized elsewhere4,19 but include abnormal levels of coagulation factors such as protein C, protein S, and antithrombin; lipoprotein(a) [Lp(a)]; homocysteine; factors VII, IX and XI; and lupus anticoagulant and antiphospholipid antibodies (APAs). Commonly explored prothrombotic genetic changes include factor V Leiden (FVL), factor II G20210A (FII), and methylenetetrahydrofolate reductase C677T (MTHFR). These approaches are reasonable, based on substantial evidence of associations with other thrombotic diseases in adults and children. Disease-specific associations of prothrombotic disorders have been suggested for childhood ischemic stroke.19 However, the same levels of evidence do not yet exist for children with perinatal stroke. In addition to the limitations noted is the known complexity of the human coagulation system and its dynamic evolution through conceptual and early postnatal development.20,21
Understanding the role of thrombophilia in perinatal stroke carries multiple clinical implications. As possible risk factors, elucidating associations may help explain distinct pathophysiological mechanisms for arterial and venous subtypes of perinatal stroke. Although perinatal stroke recurrence is uncommon (< 1% to 2%), it has been associated with thrombophilia22 and the issue of which, if any, child should be anticoagulated is often raised. Finally, the question of testing children with perinatal stroke for thrombophilia later in life has not been directly addressed. Implications include unnecessary anxiety for families, lack of informed treatment indications, accurate clinical best practice guidelines, and efficient health care resource use for the nearly 100 000 North American families currently affected by perinatal stroke.
We conducted a population-based, prospective, single-center, case-control study of children with MRI-defined perinatal stroke diseases to explore associations with known prothrombotic conditions.
Methods
Populations
Patients with perinatal ischemic stroke were identified through the Alberta Perinatal Stroke Project.23 Originating in 2008, this population-based registry systematically identified all children in southern Alberta (population ∼2.2 million, ∼26 000 live births per year), Canada, with perinatal stroke. Retrospectively (1998-2008), case ascertainment methods including exhaustive International Classification of Diseases, ninth and 10 revisions, code searching and medical record reviews identified all potential cases. Prospectively (2007-2015), children were identified at the time of NAIS, APPIS, or PVI diagnosis. Recruitment occurred by consultation to the Alberta Children’s Hospital Pediatric Stroke Service or referral to the Alberta Children’s Hospital Perinatal Stroke Clinic, where all participants were reviewed and examined by a stroke neurologist to confirm diagnosis and arrange definitive MRI if not already available. Patients were classified as NAIS, APPIS, or PVI based on clinical history and imaging results as described previously.2 Inclusion criteria were: (1) MRI-confirmed ischemic perinatal stroke; (2) no identifiable high-likelihood etiology for stroke, such as congenital heart disease or bacterial meningitis; and (3) informed consent/assent.
Control participants were recruited through an established healthy participant program. Children undergoing general anesthesia for routine elective procedures were screened preoperatively. Those without chronic medical conditions were contacted before procedure to confirm health and absence of any medications or first-degree relatives with clotting disorders. Controls were recruited randomly from eligible participants while maintaining a balance of age and sex comparable to the stroke group. At onset of anesthesia, a blood sample was collected and transported to the institutional special coagulation laboratory per protocol.
Thrombophilia evaluations
Prothrombotic evaluations were conducted prospectively. Laboratory testing of prothrombotic conditions in perinatal stroke studies have been inconsistent, including different methodologies and timing during development. Because most evaluations are sensitive to these factors, a standardized approach was developed to optimize consistency in a population typically diagnosed between birth and 12 months. Blood draws were performed at 12 ± 2 months of age for all cases diagnosed in the first year of life. Those diagnosed at a later age were tested at the time of study enrollment. Genetic testing was performed on the same samples. Methods were approved by the institutional research ethics board.
Prothrombotic outcomes were collected and measured according to standardized operating procedures. Peripheral blood was collected into vacutainer tubes (2.7 mL) containing 3.2% sodium citrate (0.105 mol/L; BD, Mississauga, Canada) and then centrifuged at room temperature for 15 minutes at 2800 RCF. The following tests were performed on an ACL TOP analyzer at the Special Coagulation Laboratory, Calgary Laboratory Services. Prothrombin time (international normalized ratio) and activated partial thromboplastin time were clot-based one-stage assays. Factor VIII, IX, and XI levels were activated partial thromboplastin time clot-based assays. Protein C test was performed on both clot-based and chromogenic-based assays. Quantitative free protein S tests were performed by latex ligand immunoassay. Protein S activity was measured by clot-based and/or chromogenic-based assays. Antithrombin quantitative test was performed by chromogenic-based assay. Fibrinogen was quantified using Clauss method. Lupus anticoagulants used a panel test including diluted Russell’s viper venom test screen, diluted Russell’s viper venom test confirmation test, and tissue thromboplastin inhibition test.
Lp(a), homocysteine, and anticardiolipin antibody and β-glycoprotein tests were performed at the chemistry laboratory of Calgary Laboratory Services. Homocysteine levels were determined using a chemiluminescent microparticle immunoassay. Lp(a) was quantified using a particle-enhanced immunoturbidimetric assay (Roche Integra). An upper limit of normal of 0.30 g/L was also applied as the most commonly used risk threshold.
Homocysteine was measured using a 1-step chemiluminescent microparticle immunoassay (Abbott Architect). APAs and β2 glycoprotein were measured using multiplex flow immunoassay Bio-Rad BioPlex 2200. Qualitative categorization of APAs as borderline or low was repeated a minimum of 8 weeks later.
All cases were tested for the genetic variants FVL, FII G20210A, and MHTFR C677T. FVL and FII mutations were detected using heminested allele-specific polymerase chain reaction (PCR). The PCR assay was carried out on microliter volumes of whole, unfractionated EDTA or citrate anticoagulated blood specimens using nested, allele-specific primers in parallel reactions. The presence of allele-specific products for 1691G- or 1691A-containing sequence in addition to a constant fragment produced from the outer primer set was indicative of the patient genotype at the factor V 1691 locus. The MTHFR polymorphism analysis was conducted by PCR and restriction enzyme digestion (nonradioactive method). The PCR assay was carried out on purified DNA extracted from nucleated blood cells (MH 29.0), and the resulting amplified product was digested overnight with Hinf I restriction enzyme. The digested products were separated on thin polyacrylamide gels, and the pattern of fragments developed by staining with ethidium bromide and visualization with UV light. The genetic prothrombotic abnormalities of interest were already well characterized in the general population with studies having high power and ethnic diversity. Therefore, a literature search was performed in Medline for population-based investigations of mutations and variants in prothrombotic factors, including FVL, FII, and MTHFR. Studies were considered if they included populations that would be similar to our sample (North American, varied ethnicity) and specified the numbers of patients and allele frequencies.
Analysis
For continuous outcomes, mean values were compared between each stroke type and controls (analysis of variance, post hoc Tukey). Potential associations between age and continuous variables was tested (Pearson or Spearman), and group comparisons were corrected for age when present (analysis of covariance). Proportions of patients falling outside the normal range (defined as 5% to 95% of control values) were compared between stroke groups (χ2/Fisher’s exact test). Frequency of genetic abnormalities was compared between stroke groups (χ2/Fisher’s exact test) and the literature-based estimates of population prevalence (z test). Analysis was repeated, grouping all arterial patients together (NAIS, APPIS). Analysis was performed using Statistical Package for the Social Sciences (version 19.0; SPSS Inc, Chicago, IL).
An initial sample-size calculation based on clinically significant effect size of >20% incidence of thrombophilia in stroke cases, power of 90%, and type I error of 0.05 suggested a sample of 32 children per group.
Results
There were 182 children with confirmed perinatal stroke potentially meeting criteria: 72 with PVI (40%), 61 with NAIS (34%), and 49 with APPIS (27%). From this sample, 135 (74%; PVI, 55; NAIS, 46; APPIS, 34) were included in this analysis. Those excluded had a presumed alternative mechanism for their stroke (meningitis, congenital cardiac disease) or incomplete results, or the parents declined testing. The control population consisted of 77 participants, resulting in a total population of 212. There were no differences in age or sex between groups except among those with NAIS, in whom median age was lower. Participant characteristics are summarized in Table 1.
Population characteristics
| . | NAIS . | APPIS . | PVI . | Control . |
|---|---|---|---|---|
| Number | 46 | 34 | 55 | 77 |
| Male (%) | 28 (61) | 14 (41) | 36 (65) | 45 (58) |
| Median age (range), years | 1.8 (0.8-17.9) | 10.3 (0.8-17.9) | 6.5 (0.9-19.1) | 5.4 (0.9-17.6) |
| . | NAIS . | APPIS . | PVI . | Control . |
|---|---|---|---|---|
| Number | 46 | 34 | 55 | 77 |
| Male (%) | 28 (61) | 14 (41) | 36 (65) | 45 (58) |
| Median age (range), years | 1.8 (0.8-17.9) | 10.3 (0.8-17.9) | 6.5 (0.9-19.1) | 5.4 (0.9-17.6) |
The final sample size, sex, and distributions are shown for the perinatal stroke populations of NAIS, APPIS, and PVI and controls. Groups were comparable, except median age in the NAIS group was lower.
To establish reference ranges for our laboratory methods, control data were analyzed and summarized as shown in Table 2. The means, standard errors, and 95% confidence intervals (CIs) are displayed for each coagulation parameter measured as a continuous variable. No age or sex differences were observed. Results were also similar to existing laboratory reference ranges (based on adults >16 years of age) and previously published values.24
Normative control values
| . | Mean . | Standard error . | 95% CI . | Laboratory reference range . |
|---|---|---|---|---|
| PT, s | 11.9 | 0.10 | 10.6-13.5 | 10.2-13.1 |
| INR | 1.1 | 0.01 | 1.0-1.2 | 0.9-1.1 |
| PTT, s | 32.8 | 0.58 | 28.3-37.5 | 27.0-37.0 |
| Fibrinogen, g/L | 2.4 | 0.06 | 1.7-3.3 | 1.4-4.1 |
| Antithrombin, U/mL | 1.03 | 0.013 | 0.86-1.21 | 0.078-1.24 |
| Protein C activity, U/mL | 0.87 | 0.025 | 0.52-1.27 | 0.67-1.26 |
| Protein S activity, U/mL | 0.87 | 0.019 | 0.64-1.11 | 0.71-1.42 |
| Protein S free, U/mL | 0.83 | 0.016 | 0.63-1.10 | 0.65-1.22 |
| Factor VIII, U/mL | 1.00 | 0.034 | 0.60-1.53 | 0.54-1.47 |
| Factor IX, U/mL | 0.76 | 0.018 | 0.54-1.07 | 0.55-1.6 |
| Factor XI, U/mL | 1.15 | 0.032 | 0.77-1.57 | 0.55-1.38 |
| Lp(a), g/L | 0.23 | 0.039 | 0.00-0.73 | <0.3 |
| . | Mean . | Standard error . | 95% CI . | Laboratory reference range . |
|---|---|---|---|---|
| PT, s | 11.9 | 0.10 | 10.6-13.5 | 10.2-13.1 |
| INR | 1.1 | 0.01 | 1.0-1.2 | 0.9-1.1 |
| PTT, s | 32.8 | 0.58 | 28.3-37.5 | 27.0-37.0 |
| Fibrinogen, g/L | 2.4 | 0.06 | 1.7-3.3 | 1.4-4.1 |
| Antithrombin, U/mL | 1.03 | 0.013 | 0.86-1.21 | 0.078-1.24 |
| Protein C activity, U/mL | 0.87 | 0.025 | 0.52-1.27 | 0.67-1.26 |
| Protein S activity, U/mL | 0.87 | 0.019 | 0.64-1.11 | 0.71-1.42 |
| Protein S free, U/mL | 0.83 | 0.016 | 0.63-1.10 | 0.65-1.22 |
| Factor VIII, U/mL | 1.00 | 0.034 | 0.60-1.53 | 0.54-1.47 |
| Factor IX, U/mL | 0.76 | 0.018 | 0.54-1.07 | 0.55-1.6 |
| Factor XI, U/mL | 1.15 | 0.032 | 0.77-1.57 | 0.55-1.38 |
| Lp(a), g/L | 0.23 | 0.039 | 0.00-0.73 | <0.3 |
Mean, variance, and 95% confidence intervals are shown for all quantified variables. Existing reference ranges from our laboratory based on testing in adolescents and adults are provided for comparison
INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time.
The results of all quantified prothrombotic measures are shown for stroke and control participants in Table 3. Mean values, variances, and 95% CIs were comparable for most measures. This did not change when NAIS and APPIS were analyzed individually or collectively. Prothrombin time was significantly shorter in AIS (11.3 ± 0.1; 95% CI, 10.1-12.6) compared with controls (11.9 ± 0.1; 95% CI, 10.6-13.5; P < .001). The international normalized ratio was also higher in controls (1.10 ± 0.01; 95% CI, 1.0-1.2) compared with both stroke groups (1.00 ± 0.01; 95% CI, 0.9-1.1; P < .001). Two additional significant results were suggested on the initial analysis of variance without correction for multiple comparisons. However, neither was in the direction expected for thrombophilia, including a lower level of antithrombin and higher level of factor XI in controls compared with either stroke group.
Quantified prothrombotic factor group comparisons
| . | PVI . | AIS . | Control . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SE . | 95% CI . | Mean . | SE . | 95% CI . | Mean . | SE . | 95% CI . | ||
| PT, s | 11.8 | 0.12 | 10.3-13.3 | 11.3 | 0.09 | 10.1-12.6 | 11.9 | 0.10 | 10.6-13.5 | <.001* |
| INR | 1.0 | 0.01 | 0.9-1.1 | 1.0 | 0.01 | 0.9-1.1 | 1.1 | 0.01 | 1.0-1.2 | <.001* |
| PTT, s | 31.9 | 0.42 | 27.9-37.7 | 31.6 | 0.39 | 27.1-38.0 | 32.8 | 0.58 | 28.3-37.5 | .18 |
| Fibrinogen, g/L | 2.5 | 0.07 | 1.8-3.3 | 2.5 | 0.07 | 1.7-3.8 | 2.4 | 0.06 | 1.7-3.3 | .51 |
| Antithrombin, U/mL | 1.06 | 0.020 | 0.88-1.28 | 1.09 | 0.016 | 0.86-1.30 | 1.03 | 0.013 | 0.86-1.21 | .018* |
| Protein C, U/mL | 0.87 | 0.027 | 0.63-1.24 | 0.87 | 0.025 | 0.51-1.22 | 0.87 | 0.025 | 0.52-1.27 | .99 |
| Protein S activity, U/mL | 0.85 | 0.022 | 0.57-1.13 | 0.86 | 0.020 | 0.60-1.20 | 0.87 | 0.019 | 0.64-1.11 | .83 |
| Protein S free, U/mL | 0.86 | 0.021 | 0.60-1.10 | 0.85 | 0.018 | 0.55-1.09 | 0.83 | 0.016 | 0.63-1.10 | .48 |
| Factor VIII, U/mL | 1.04 | 0.046 | 0.58-1.69 | 1.00 | 0.032 | 0.64-1.47 | 1.00 | 0.034 | 0.60-1.53 | .72 |
| Factor IX, U/mL | 0.77 | 0.018 | 0.60-1.01 | 0.80 | 0.023 | 0.54-1.17 | 0.76 | 0.018 | 0.54-1.07 | .32 |
| Factor XI, U/mL | 1.00 | 0.037 | 0.62-1.39 | 1.02 | 0.033 | 0.69-1.58 | 1.15 | 0.032 | 0.77-1.57 | .004* |
| Lp(a), g/L | 0.24 | 0.040 | 0.00-0.69 | 0.24 | 0.037 | 0.00-0.91 | 0.23 | 0.039 | 0.00-0.73 | .989 |
| . | PVI . | AIS . | Control . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SE . | 95% CI . | Mean . | SE . | 95% CI . | Mean . | SE . | 95% CI . | ||
| PT, s | 11.8 | 0.12 | 10.3-13.3 | 11.3 | 0.09 | 10.1-12.6 | 11.9 | 0.10 | 10.6-13.5 | <.001* |
| INR | 1.0 | 0.01 | 0.9-1.1 | 1.0 | 0.01 | 0.9-1.1 | 1.1 | 0.01 | 1.0-1.2 | <.001* |
| PTT, s | 31.9 | 0.42 | 27.9-37.7 | 31.6 | 0.39 | 27.1-38.0 | 32.8 | 0.58 | 28.3-37.5 | .18 |
| Fibrinogen, g/L | 2.5 | 0.07 | 1.8-3.3 | 2.5 | 0.07 | 1.7-3.8 | 2.4 | 0.06 | 1.7-3.3 | .51 |
| Antithrombin, U/mL | 1.06 | 0.020 | 0.88-1.28 | 1.09 | 0.016 | 0.86-1.30 | 1.03 | 0.013 | 0.86-1.21 | .018* |
| Protein C, U/mL | 0.87 | 0.027 | 0.63-1.24 | 0.87 | 0.025 | 0.51-1.22 | 0.87 | 0.025 | 0.52-1.27 | .99 |
| Protein S activity, U/mL | 0.85 | 0.022 | 0.57-1.13 | 0.86 | 0.020 | 0.60-1.20 | 0.87 | 0.019 | 0.64-1.11 | .83 |
| Protein S free, U/mL | 0.86 | 0.021 | 0.60-1.10 | 0.85 | 0.018 | 0.55-1.09 | 0.83 | 0.016 | 0.63-1.10 | .48 |
| Factor VIII, U/mL | 1.04 | 0.046 | 0.58-1.69 | 1.00 | 0.032 | 0.64-1.47 | 1.00 | 0.034 | 0.60-1.53 | .72 |
| Factor IX, U/mL | 0.77 | 0.018 | 0.60-1.01 | 0.80 | 0.023 | 0.54-1.17 | 0.76 | 0.018 | 0.54-1.07 | .32 |
| Factor XI, U/mL | 1.00 | 0.037 | 0.62-1.39 | 1.02 | 0.033 | 0.69-1.58 | 1.15 | 0.032 | 0.77-1.57 | .004* |
| Lp(a), g/L | 0.24 | 0.040 | 0.00-0.69 | 0.24 | 0.037 | 0.00-0.91 | 0.23 | 0.039 | 0.00-0.73 | .989 |
Mean, standard error, and 95% CI for each continuous variable outcome are shown for PIV, AIS (comprising NAIS and APPIS), and controls. P value reflects significance of the original analysis of variance without correction for multiple comparisons.
INR, international normalized ratio; PT prothrombin time; PTT partial thromboplastin time.
Significant pairwise difference.
Using the ranges of normal established by the control sample, and considering the expected direction of abnormality associated with pathological thrombosis, the frequency of abnormalities was not different for stroke patients. This did not differ if NAIS and APPIS were analyzed individually or as a single group. These results are summarized in Table 4.
Frequency of abnormal results across groups
| . | No. (%) . | ||
|---|---|---|---|
| PVI . | AIS . | Control . | |
| PT | 1 (2) | 4 (5) | 1 (1) |
| INR | 0 | 1 (1) | 0 |
| PTT | 4 (8) | 6 (8) | 3 (4) |
| Fibrinogen | 0 | 1 (1) | 0 |
| Antithrombin | 0 | 1 (1) | 1 (1) |
| Protein C | 5 (10) | 7 (9) | 10 (14) |
| Protein S | 9 (18) | 9 (12) | 10 (14) |
| Protein S free | 9 (17) | 12 (16) | 18 (24) |
| Factor VIII | 4 (8) | 4 (6) | 5 (7) |
| Factor IX | 0 | 0 | 0 |
| Factor XI | 3 (6) | 8 (11) | 12 (16) |
| Lp(a) | 0 | 0 | 0 |
| Anticardiolipin antibody* | 5 (10) | 7 (10) | 2 (3) |
| Lupus inhibitor | 0 | 0 | 1 (1) |
| . | No. (%) . | ||
|---|---|---|---|
| PVI . | AIS . | Control . | |
| PT | 1 (2) | 4 (5) | 1 (1) |
| INR | 0 | 1 (1) | 0 |
| PTT | 4 (8) | 6 (8) | 3 (4) |
| Fibrinogen | 0 | 1 (1) | 0 |
| Antithrombin | 0 | 1 (1) | 1 (1) |
| Protein C | 5 (10) | 7 (9) | 10 (14) |
| Protein S | 9 (18) | 9 (12) | 10 (14) |
| Protein S free | 9 (17) | 12 (16) | 18 (24) |
| Factor VIII | 4 (8) | 4 (6) | 5 (7) |
| Factor IX | 0 | 0 | 0 |
| Factor XI | 3 (6) | 8 (11) | 12 (16) |
| Lp(a) | 0 | 0 | 0 |
| Anticardiolipin antibody* | 5 (10) | 7 (10) | 2 (3) |
| Lupus inhibitor | 0 | 0 | 1 (1) |
Using the established normative values and direction of abnormality favoring thrombophilia, the proportion of participants with abnormal results are shown. No differences were observed between groups, including individual analysis of NAIS and APPIS populations.
INR, international normalized ratio; PT prothrombin time; PTT partial thromboplastin time.
Anticardiolipin results shown are from the original test and were normal on repeat testing in 86%.
APAs were detected at low levels in 14 participants (7%) across all groups: anticardiolipin antibodies in 5 patients with PVI (borderline, 1; low positive, 4), 7 patients with AIS (borderline, 1; low, 5; moderate, 1), and 2 control participants (borderline, 1; low positive, 1). However, repeat testing at least 8 weeks later in 13 of the 14 participants with borderline or low levels revealed negative results in 11 and borderline in 2 (control, 1; PVI, 1). Lupus inhibitor was found in only 1 control participant.
The results of the genetic analyses are shown in Table 5. The prevalence of FVL heterozygosity was 10.7% in the PVI, 6.3% in the NAIS, and 8.1% in the APPIS groups. These proportions were not significantly different from one another. Rates for all arterial (NAIS, APPIS) were comparable to those for PVI. These rates did not differ from the estimated published North American population rate of 6%.25 One child with PVI was homozygous for FVL, which was not distinguishable from chance against the extremely low population rate of 0.1%.26 Factor II heterozygous mutation was found in 5.4% of PVI cases, which did not differ from 2.2% and 2.9% of patients with NAIS and APPIS, respectively. Compared with all arterial, the rate of factor II mutations was not higher in patients with PVI (5.4% vs 2.4%; P = .18). Compared with the published population value of 3.8%,27 rates were comparable in NAIS, APPIS, and PVI (P = .2).
Thrombophilia genotypes
| . | PVI . | NAIS . | APPIS . | Arterial . | Population . |
|---|---|---|---|---|---|
| FVL | |||||
| Heterozygous | 5 | 3 | 3 | 6 | |
| Homozygous | 1 | 0 | 0 | 0 | |
| Wild type | 49 | 43 | 32 | 75 | |
| Mutation, % | 10.7 | 6.3 | 8.1 | 7.2 | 6 |
| 95% CI | 1.5-16.7 | −0.7-17.9 | −0.7-17.8 | 1.70-13.1 | |
| Prothrombin 20210A | |||||
| Heterozygous | 3 | 1 | 1 | 2 | |
| Wild type | 52 | 45 | 34 | 79 | |
| Mutation, % | 5.4 | 2.2 | 2.9 | 2.4 | 3.8 |
| 95% CI | −0.55-11.5 | −2.0-6.4 | −2.6-8.4 | −0.91-5.85 | |
| MTHFR | |||||
| Homozygous TT | 6 | 4 | 3 | 7 | |
| Mutation, % | 11.7 | 9.3 | 9.1 | 9.2* | 15 |
| 95% CI | 2.92-20.6 | 0.62-18.0 | −0.7-18.9 | 2.7-15.7 | |
| Heterozygous CT | 21 | 16 | 13 | 29 | |
| Mutation, % | 41.1 | 37.2 | 39.3 | 38.1 | 37 |
| 95% CI | 27.6-54.7 | 22.8-51.7 | 22.7-56.1 | 27.2-49.1 | |
| Homozygous CC | 24 | 23 | 17 | 40 | |
| Mutation, % | 47 | 53.4 | 51.5 | 52.6 | 57 |
| 95% CI | 33.4-60.8 | 38.6-68.4 | 34.4-68.6 | 41.4-63.9 |
| . | PVI . | NAIS . | APPIS . | Arterial . | Population . |
|---|---|---|---|---|---|
| FVL | |||||
| Heterozygous | 5 | 3 | 3 | 6 | |
| Homozygous | 1 | 0 | 0 | 0 | |
| Wild type | 49 | 43 | 32 | 75 | |
| Mutation, % | 10.7 | 6.3 | 8.1 | 7.2 | 6 |
| 95% CI | 1.5-16.7 | −0.7-17.9 | −0.7-17.8 | 1.70-13.1 | |
| Prothrombin 20210A | |||||
| Heterozygous | 3 | 1 | 1 | 2 | |
| Wild type | 52 | 45 | 34 | 79 | |
| Mutation, % | 5.4 | 2.2 | 2.9 | 2.4 | 3.8 |
| 95% CI | −0.55-11.5 | −2.0-6.4 | −2.6-8.4 | −0.91-5.85 | |
| MTHFR | |||||
| Homozygous TT | 6 | 4 | 3 | 7 | |
| Mutation, % | 11.7 | 9.3 | 9.1 | 9.2* | 15 |
| 95% CI | 2.92-20.6 | 0.62-18.0 | −0.7-18.9 | 2.7-15.7 | |
| Heterozygous CT | 21 | 16 | 13 | 29 | |
| Mutation, % | 41.1 | 37.2 | 39.3 | 38.1 | 37 |
| 95% CI | 27.6-54.7 | 22.8-51.7 | 22.7-56.1 | 27.2-49.1 | |
| Homozygous CC | 24 | 23 | 17 | 40 | |
| Mutation, % | 47 | 53.4 | 51.5 | 52.6 | 57 |
| 95% CI | 33.4-60.8 | 38.6-68.4 | 34.4-68.6 | 41.4-63.9 |
Proportions of each genotype for FVL, prothrombin gene 20210A, and MTHFR are shown for each perinatal stroke disease. Control prevalence is estimated from the literature. No differences were observed between groups except all arterial had significantly lower occurrence of the TT genotype (P = .04). Ten patients (PVI, 5; NAIS, 3; APPIS, 2) did not have MTHFR results available. Homocysteine levels did not differ by stroke group or genotype.
P = .04, all arterial lower than PVI.
No difference was observed in the distribution of MTHFR genotypes between stroke subgroups. There were fewer patients with all arterial carrying the homozygous TT genotype compared with the population (9.1% vs 15%; P = .04).28 No participants had elevated serum homocysteine. The mean homocysteine was 5.8 ± 0.27 umol/L in PVI, 6.7 ± 0.33 umol/L in APPIS, and 5.5 ± 0.19 umol/L in NAIS. In a sample of 32 healthy controls tested in the same laboratory, mean values, variance, and range were similar at 5.94 ± 0.25 umol/L. These values fell within our laboratory reference range for ages 16 to 49 years of 4.9 to 13.3 umol/L (males) and 4.1 to 9.1 umol/L (females). Associations between MTHFR and homocysteine demonstrated the expected trend, although this did not reach statistical significance (CC, 5.85 ± 0.22; CT, 6.06 ± 0.26; TT, 6.42 ± 0.59).
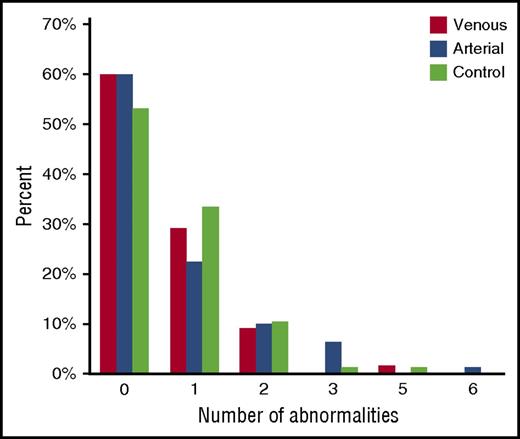
The number of abnormal test results did not differ between stroke subtypes and controls (Figure 1). No abnormalities were detected in 41 control participants (53%), 33 patients with PVI (60%), and 48 patients with AIS (60%; NAIS, 25; APPIS, 23). One abnormality was found in 26 controls (34%), 16 patients with PVI (29%), and 18 patients with AIS (23%). Five or 6 abnormalities were detected in 1 each of the control, PVI, and AIS groups.
Comparison by number of prothrombotic abnormalities. Relative proportions of participants having each of none through 6 abnormalities are shown by stroke group or controls. Most participants had no abnormalities. No difference was found between stroke groups and controls regardless of number of abnormalities.
Comparison by number of prothrombotic abnormalities. Relative proportions of participants having each of none through 6 abnormalities are shown by stroke group or controls. Most participants had no abnormalities. No difference was found between stroke groups and controls regardless of number of abnormalities.
Discussion
Our results suggest that patients with perinatal stroke undergoing thrombophilia investigations in childhood demonstrate minimal risk of harboring a prothrombotic disorder compared with the general population. Our findings disagree with those of previous studies, but our methodology carries substantial additional strengths, including specific, MRI-classified perinatal stroke disease states; prospective, case-control data within the same laboratory; and a well-powered, population-based sample.
Perhaps the most compelling association between thrombophilia and perinatal stroke previously described is that of FVL. Being a commonly performed genetic test with established population prevalence makes previous reports of much higher than expected rates seem significant.29-32 As a constant genetic factor present from birth, FVL also avoids potentially complicating, confounding issues of neonatal versus childhood testing and developmental hemostasis. However, our results demonstrated rates of FVL (as well as prothrombin gene mutations) comparable to those in the general population. Prevalence among 4047 participants in the Physician’s Health Survey and Women’s Health Study with no history of thrombosis varied from 0.45% in Asian Americans to 5.27% in whites, with a pooled frequency of 3.7%.25 Although our results cannot refute all previous studies, the clinical significance of FVL heterozygosity should also be considered. With >95% of such individuals never incurring a serious thrombotic disease during their lifetime, the benefits of testing children with perinatal stroke is questionable, even if rates are slightly increased.
Additional considerations exist for the contrast between our results and those of previous studies. The possibility of selection bias can be considered, with many previous positive studies coming from specialized thrombosis centers where the advantages of methodological and other expertise may be complicated by referral patterns, leading to higher rates of thrombophilia. Examination of the limited case-control studies to date supports this possibility, with nearly 1 in 4 so-called normal participants having at least 1 prothrombotic abnormality.33 Second, many previous studies used reference ranges or cutoffs established elsewhere. For example, many studies of Lp(a) used a cutoff of >0.3 mg/dL.34,35 In contrast, our results found that the normal range in controls extended far above this number, with no differences observed between cases and controls. Finally, another highly powered study of perinatal stroke thrombophilia submitted for publication also describes a similar lack of association between known thrombophilias and perinatal stroke (L. Lehman, personal communication, 11 October 2016).
The MTHFR story also merits consideration. This common genotype has been repeatedly associated with thrombosis over decades, including common descriptions in perinatal stroke.4 MTHFR prevalence varies among ethnic groups, from 8% to 30% for the TT genotype; in >1000 newborns, the North American genotype distribution was 57%, 37%, and 15% for CC, CT, and TT genotypes, respectively.36 The association of MTHFR mutations with stroke and thrombosis not only is inconsistent but has virtually been disproven to the point of new guidelines suggesting testing is no longer warranted.37 The predominance of this so-called genetic thrombophilia in the literature for so long across so many studies may highlight the complexities of defining such relationships and the high risk of false-positive associations.
The potential association between perinatal stroke and APAs is also becoming clearer. Although multiple studies have suggested this association,5,22,29,38-40 there have been no well-powered, population-based, case-control investigations. Importantly, none has confirmed that positive findings are both strong and persistent over time. The most common cause of a borderline or low positive test is probably intercurrent illness. It has clearly been shown in recent meta-analyses that only such persistently abnormal positivity is associated with incident thromboembolic disease in children.41 A recent prospective study followed 12 of 62 children with NAIS or neonatal cerebral sinus venous thrombosis who had persistently positive acute APAs.42 Followed with serial testing for a median of 3.5 years, many were anticoagulated, but 10 of 12 demonstrated normalization of APA status, and there were no recurrences. In contrast, our study, which included only those children with persistent, significant APA positivity, found very low prevalence rates and no association with specific perinatal stroke disease states. The role of maternal APAs is less clear; however, the few studies suggesting an association29,43-47 have the same limitations described earlier in this section, and the single best acute case-control study in NAIS found no association with APA.33 That maternal APA positivity has been disassociated from infant positivity in NAIS,42 and that it is not readily apparent how maternal thrombophilia would lead to cerebral thromboembolism in the fetus or newborn, further calls into question the relevance of maternal APA status. With known risks of APA syndrome and available treatments, this issue is of great clinical relevance. However, the body of evidence and our new results here suggest APAs are unlikely to influence the health of children who have experienced a perinatal stroke in the past.
Despite accounting for a majority of perinatal stroke cases, presumed perinatal stroke has been relatively neglected in the literature, with no case-control studies to date. In fact, most early studies did not even specify between perinatal stroke types. The one exception was an uncontrolled Turkish study of 35 participants suggesting a high rate of thrombophilia.18 However, the most common finding was MTFHR, whereas other more legitimate abnormalities were uncommon. Our study is the first to add MRI-confirmed, disease-specific, population-based, case-control data. Testing nearly 90 cases of presumed perinatal stroke compared with controls within the same laboratory, we found essentially no differences. We believe this provides the best evidence to date and suggests that childhood thrombophilia is uncommon in presumed perinatal stroke.
The lack of association we observed between prothrombotic abnormalities and perinatal stroke does not preclude a role for disordered coagulation in the pathogenesis of perinatal stroke. Could acute changes be more important? One of the best acute NAIS studies to date prospectively performed a thrombophilia panel similar to ours in 91 consecutive term-born cases matched 2:1 to healthy controls.33 Important considerations are that acutely ill neonates were not exclude,d including those with asphyxia and infections. Blood was collected between 3 weeks and 6 months of age (controls were from 6-16 weeks of age). Multiple moderately powered case-control studies of clinical variables associated with NAIS have failed to define consistent associations supportive of causation.7-10 They have agreed, however, that multifactorial pathogenesis is likely often required and that thromboembolism is almost certainly the culprit in arterial perinatal stroke. Placental disease leading to fetal cerebral embolism is a leading candidate mechanism, supported by associations with placental disease, common bilateral lesions without cardiac abnormality, and virtually no recurrence risk (<1% to 2%).4,48 A low-flow vascular bed prone to thrombosis such as an inflamed placenta in chorioamnionitis could certainly theoretically interact with a neonatal thrombotic state to lead to perinatal stroke. Although less studied, abnormal thrombosis may also be associated with PVI mechanisms.15 Such acute or transient alterations in thrombotic state are also supported by previous reports of acute protein C and S defects typically resolving at follow-up in children with NAIS.33 Our results therefore do not alter the strong suspicion of acutely disturbed thrombosis in perinatal stroke.
Additional limitations are acknowledged. We did not test the mothers of children, although even studies performed acutely have shown inconsistent results,29,44 with potential abnormalities not persisting to the chronic phase.42 We did not explore previous suggestions of association between thrombophilia and adverse outcome, primarily for lack of recurrence in our population and no other rationale to associate blood clotting with outcome.17,49 Several recently described thrombophilias potentially associated with stroke in children were not included in our panel, including plasminogen activator inhibitor-1 4G6755G, ADAMTS13, and plasma glutathione peroxidase.50-52 Importantly, our study of specific, idiopathic perinatal stroke diseases does not exclude possible associations with other diseases (eg, cerebral sinovenous thrombosis) or major risk factors such as congenital heart disease.
Our results carry clinical implications. With minimal association and virtually no risk of stroke recurrence, we suggest that routine testing in childhood is not required. Not only is there little if any apparent benefit, but there may also be substantial risk of harm to children and their families. Completing ≥15 tests will most often lead to at least 1 seemingly abnormal result. This in turn may lead to unnecessary additional testing and referrals to specialists, with implications for child and family anxiety as well as health care resource use. These same issues have been addressed in adult stroke,53 with conclusions similar to ours: “If there is an association between thrombophilia and arterial stroke, then it is a weak one… The consequences of ordering these tests and attributing causality to an arterial event can result in significant costs to the health care system and pose a potential risk to patients, because this may lead to inappropriate use of long-term oral anticoagulants, exposing patients to harm without a clearly defined benefit.” We have not addressed the role of acute testing. However, with interpretation being complex, few if any clear indications for anticoagulation or other alterations in early management, and almost no risk of recurrence, the utility of acute testing is questionable. We have also not addressed specific circumstances such as a strong family history of pathological thrombosis.
In conclusion, thrombophilia in children with ischemic perinatal stroke is rare, with rates approximating those in the normal population, and routine testing in childhood is not indicated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The assistance of Robin Cox and the Division of Pediatric Anesthesia at the Alberta Children's Hospital in facilitating the collection of control blood samples is gratefully acknowledged.
This work was supported by Alberta Innovates Health Solutions, the Heart and Stroke Foundation of Canada, and NeuroDevNet.
Authorship
Contribution: C.C. contributed to participant recruitment, disease classification, data collection and management, statistical analysis, and manuscript writing; A.M. contributed to case identification and ascertainment, participant recruitment, disease classification, data collection and management, statistical analysis, and manuscript editing; P.M. and M.L. contributed expertise in pediatric thrombophilia, including methods development, laboratory methods, tests performed, data analysis, and manuscript editing; X.Y.J. contributed expertise in laboratory methods for thrombophilia testing, including methods development, results analysis, and manuscript editing; A.F. contributed to case and control participant recruitment, performed all control sample acquisition, and contributed to quality assurance for laboratory methods, data collection and management, and manuscript editing; and A.K. was the principal investigator, contributing to all elements outlined above as well as funding, trainee supervision, and final manuscript editing.
Conflict-of-interest disclosure: P.M. served on the steering committee for Bayer, on the clinical events committee for Xeltis, and as a site investigator for Pfizer and Daiichi Sankyo. The remaining authors declare no competing financial interests.
Correspondence: Adam Kirton, Alberta Children’s Hospital, Room C1-320, 2888 Shaganappi Trail NW, Calgary, AB, Canada T3B6A8; e-mail: adam.kirton@ahs.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal