Abstract
Hemolytic uremic syndrome (HUS) is a thrombotic microangiopathy characterized by intravascular hemolysis, thrombocytopenia, and acute kidney failure. HUS is usually categorized as typical, caused by Shiga toxin–producing Escherichia coli (STEC) infection, as atypical HUS (aHUS), usually caused by uncontrolled complement activation, or as secondary HUS with a coexisting disease. In recent years, a general understanding of the pathogenetic mechanisms driving HUS has increased. Typical HUS (ie, STEC-HUS) follows a gastrointestinal infection with STEC, whereas aHUS is associated primarily with mutations or autoantibodies leading to dysregulated complement activation. Among the 30% to 50% of patients with HUS who have no detectable complement defect, some have either impaired diacylglycerol kinase ε (DGKε) activity, cobalamin C deficiency, or plasminogen deficiency. Some have secondary HUS with a coexisting disease or trigger such as autoimmunity, transplantation, cancer, infection, certain cytotoxic drugs, or pregnancy. The common pathogenetic features in STEC-HUS, aHUS, and secondary HUS are simultaneous damage to endothelial cells, intravascular hemolysis, and activation of platelets leading to a procoagulative state, formation of microthrombi, and tissue damage. In this review, the differences and similarities in the pathogenesis of STEC-HUS, aHUS, and secondary HUS are discussed. Common for the pathogenesis seems to be the vicious cycle of complement activation, endothelial cell damage, platelet activation, and thrombosis. This process can be stopped by therapeutic complement inhibition in most patients with aHUS, but usually not those with a DGKε mutation, and some patients with STEC-HUS or secondary HUS. Therefore, understanding the pathogenesis of the different forms of HUS may prove helpful in clinical practice.
Introduction
Hemolytic uremic syndrome (HUS) is one of the disease processes that belong to thrombotic microangiopathies (TMAs). The common features for TMAs are microangiopathic hemolysis, thrombocytopenia, and thrombi in small vessels that lead to end organ damage. The most frequently encountered TMAs clinically are HUS associated with Shiga toxin–producing Escherichia coli infection (STEC-HUS) and thrombotic thrombocytopenic purpura (TTP), followed by atypical HUS (aHUS) and HUS with a coexisting disease (called secondary HUS).1 TMAs are also part of the pathology of other conditions such as elevated hemolysis and liver enzymes and low platelet count (collectively called HELLP syndrome),2 disseminated intravascular coagulation,3 malignant hypertension,4 antiphospholipid antibody syndrome,5 and scleroderma renal crisis.6
Within the TMA syndromes, knowledge regarding the pathogenesis of STEC-HUS and aHUS has recently increased considerably. STEC-HUS is usually initiated a few days after clinical gastroenteritis caused by STEC (usually serotype O157:H7 or O104:H4), and the Shiga toxin is central in causing endothelial cell damage, thereby apparently initiating the disease process. Understanding the central differences in pathogenesis between aHUS and STEC-HUS began with the discovery of the association between aHUS and mutations in the gene coding for the key complement regulator in plasma, complement factor H (CFH).7 Thereafter, mutations in several other complement regulators and other complement proteins, such as C3, factor B, factor I, and CD46, have been identified.8 Many patients have more than 1 mutation or rare polymorphisms affecting the complement system.9
In addition to mutations in complement proteins, some patients with aHUS have mutations also in or only in molecules not directly linked to the complement system. Those molecules include diacylglycerol kinase ε,10 plasminogen,11 and factor XII (although only in the presence of anti-factor H autoantibodies).9,12 In addition, some patients with aHUS are associated with mutations in thrombomodulin (CD141),13 which has a role in both coagulation and complement regulation.13,14
In secondary HUS, a coexisting disease such as autoimmunity, transplantation, or cancer, or an infection, normal pregnancy, or use of certain cytotoxic drugs is associated with disease manifestation.15 Pathogenesis of secondary HUS has not been intensively studied, but complement is involved in some cases. In this review, the pathogenesis of STEC-HUS, aHUS, and secondary HUS are discussed with an emphasis on the role of complement in the disease processes and the similarities and differences between STEC-HUS and aHUS, also considering the possible role of complement in secondary HUS.
Initiation of disease processes in HUS
STEC-HUS
The process of pathogenesis in typical HUS or STEC-HUS is apparently initiated when the Shiga toxin (or Shiga-like toxin), a known potent cytotoxin, binds to cell membrane glycolipid Gb3 (via domain B). Domain A is internalized and subsequently halts protein synthesis and induces apoptosis of the affected cell.16 The Shiga toxin has several additional effects on endothelial cells, one of which is enhanced expression of functional tissue factor that could contribute to microvascular thrombosis.17 The toxin causes damage to or activation of endothelium,18 red cells,19 and platelets.20 Clinically, STEC-HUS can be nearly as severe as aHUS (with mortality of up to 5%), as exemplified during the wide outbreak in Germany and some other European countries in 2011.21 The main reason why gastrointestinal infection particularly affects kidneys is thought to be the tissue tropism of Shiga-toxin on the basis of the strong expression of Gb3 on the glomerular endothelium.22
Secondary HUS
Secondary HUS is initiated by a coexisting disease or condition. The most frequently reported diseases that lead to clinically evident secondary HUS include infections, especially those caused by Streptococcus pneumoniae,23 and the influenza virus.24 These infections are considered as causes of HUS not just triggers for the disease, although the distinction between cause and trigger is not evident. In addition to infections, secondary HUS may be associated with transplantation (solid organ or bone marrow),25-28 autoimmune disease,29,30 cancer,31,32 pregnancy,33 and the use of certain cytotoxic drugs.34,35 The common feature for these coexisting diseases or conditions is that they may cause direct cell damage, promote activation of the complement system in general, or enhance activation of complement on self cells (Figure 1).
Predisposing factors, promoters, and triggers of aHUS and secondary HUS. Under physiological conditions, regulation of complement is always at a higher level than the activation challenge. (Right) Regulation consists of the role of the membrane regulators CD35, CD46, CD55, and CD59, and the plasma regulators factors H and I. Regulation is compromised by mutations in complement regulators, autoantibodies against factor H, or potentially some infections in which microbes remove sialic acids from the surface of self cells. Regulation is enhanced by upregulation of complement protein expression in liver (eg, because of acute phase reaction or pregnancy) or introduction of additional regulators by plasma exchange. (Left) Activation of complement consists of continuous alternative pathway activation and occasional (or longer standing) physiological or pathological activation. The level of complement activation can be increased by mutations in complement proteins C3 or factor B, infections, iatrogenic phenomena (eg, transplantation, plasma exchange, or certain drugs), pregnancy, or malignancy. The net result of all the activation and regulation boosting or inhibiting effects may dictate whether activation overrides regulation leading or contributing to a pathological process.
Predisposing factors, promoters, and triggers of aHUS and secondary HUS. Under physiological conditions, regulation of complement is always at a higher level than the activation challenge. (Right) Regulation consists of the role of the membrane regulators CD35, CD46, CD55, and CD59, and the plasma regulators factors H and I. Regulation is compromised by mutations in complement regulators, autoantibodies against factor H, or potentially some infections in which microbes remove sialic acids from the surface of self cells. Regulation is enhanced by upregulation of complement protein expression in liver (eg, because of acute phase reaction or pregnancy) or introduction of additional regulators by plasma exchange. (Left) Activation of complement consists of continuous alternative pathway activation and occasional (or longer standing) physiological or pathological activation. The level of complement activation can be increased by mutations in complement proteins C3 or factor B, infections, iatrogenic phenomena (eg, transplantation, plasma exchange, or certain drugs), pregnancy, or malignancy. The net result of all the activation and regulation boosting or inhibiting effects may dictate whether activation overrides regulation leading or contributing to a pathological process.
aHUS
According to the currently prevailing classification, aHUS is not associated with infections or a coexisting disease.15 Instead, it is usually associated with a genetic or acquired defect in regulation of complement activation on host cells. In a number of aHUS patients, an infection (often an upper respiratory tract infection) precedes the clinical triad typical for TMAs.8 In aHUS, infections are usually considered as triggers, not as causes of the disease as such.
Common features
The symptoms and clinical signs may be practically identical in STEC-HUS, secondary HUS, and aHUS. Another common feature is that some patients with aHUS and also STEC-HUS and secondary HUS have either mutations or autoantibodies that are associated with impaired complement regulation.19,36-38 It is also noteworthy that the same set of cells (endothelial cells, red cells, and platelets) may be damaged or activated in both STEC-HUS39 and aHUS,40,41 and apparently in secondary HUS as well. These common features indicate similarities in disease pathogenesis of these 3 forms of HUS.
Complement as a danger for plasma-exposed cells
Complement as an immune defense
Complement is activated via 3 pathways: classical, lectin, and alternative. All the pathways lead to target elimination via 2 main mechanisms, which are phagocytosis and direct lysis. The more important of these is promotion of phagocytosis via opsonization with C3b and its fragments, as well as phagocyte attraction via release of powerful chemotactic and anaphylatoxic peptides such as C5a.42 These phenomena lead to rapid opsonophagocytosis of the target by neutrophils and macrophages (Figure 2A). The second main mechanism of target elimination by complement is direct lysis of target cells via formation of membrane pores, or so-called membrane attack complexes (Figure 2A).43,44 In addition to these effects, complement also has a role in initiating and enhancing the adaptive immunity.45
Consequencesof complement activation. (A) The complement system can be activated via 3 pathways: classical, lectin, and alternative. All pathways lead to formation of powerful enzymes, the C3-convertases, followed by activation of the terminal cascade. The main effector functions of complement (promotion of opsonophagocytosis by opsonization and chemotaxis and formation of lytic membrane attack complexes) aim to destroy harmful agents such as microbes. Lysis of target cells can lead to damage-induced enhancement of complement activation. (B) Alternative pathway activation is based on continuous, low-level covalent deposition of C3b molecules onto practically all surfaces in contact with plasma. If the C3b molecule is allowed to form an enzyme (shown in red), new C3b deposits will be formed around the enzyme leading to rapid amplification of the activation. If the regulator factor H binds to C3b, the convertase enzyme is inactivated and no complement activation follows. The simultaneous interaction of factor H with both C3b and cell surface sialic acids (or possibly glycosaminoglycans [GAGs]) is essential for proper regulation on self red cells, platelets, and endothelial cells. If this fails, disbalance between activation and regulation may lead to pathogenesis of atypical HUS. iC3b, C3b molecule incapable of forming an enzyme with factor B.
Consequencesof complement activation. (A) The complement system can be activated via 3 pathways: classical, lectin, and alternative. All pathways lead to formation of powerful enzymes, the C3-convertases, followed by activation of the terminal cascade. The main effector functions of complement (promotion of opsonophagocytosis by opsonization and chemotaxis and formation of lytic membrane attack complexes) aim to destroy harmful agents such as microbes. Lysis of target cells can lead to damage-induced enhancement of complement activation. (B) Alternative pathway activation is based on continuous, low-level covalent deposition of C3b molecules onto practically all surfaces in contact with plasma. If the C3b molecule is allowed to form an enzyme (shown in red), new C3b deposits will be formed around the enzyme leading to rapid amplification of the activation. If the regulator factor H binds to C3b, the convertase enzyme is inactivated and no complement activation follows. The simultaneous interaction of factor H with both C3b and cell surface sialic acids (or possibly glycosaminoglycans [GAGs]) is essential for proper regulation on self red cells, platelets, and endothelial cells. If this fails, disbalance between activation and regulation may lead to pathogenesis of atypical HUS. iC3b, C3b molecule incapable of forming an enzyme with factor B.
Activation of complement
Most immune systems recognize targets by self molecules that bind to pathogen-associated molecular patterns (cellular immunity) or molecules specific to a certain microbe (antibody-mediated immunity). This is also true for the classical pathway of complement, which recognizes targets via binding of C1q to immunoglobulin G or M, and the lectin pathway, which recognizes glycans on the target surface via mannose-binding lectin or ficolins. Distinct from these is the alternative pathway of complement, which is based on constant and spontaneous low-level activation in plasma. This results in a low number of covalently bound (via hydroxyl or amine groups) C3b depositions on practically all biological surfaces in contact with plasma,46,47 a phenomenon that can be compared with blind shooting. The fate of the C3b deposits determines whether the surface will be destroyed or not. If the initial C3b deposit is rapidly inactivated, then activation fails to proceed and the surface is spared.
However, if C3b is not rapidly eliminated or cleared from the membrane surface, it participates in further formation of the C3 convertase (C3bBb) that generates more C3b deposits, which in turn may form similar enzymes leading to amplification and ultimately elimination of the target (Figure 2B).48
The special feature of the alternative pathway to spontaneously attack any surface provides a strategy to defend against any kind of biomaterial. This feature, however, leads to a potential to attack host cells because some C3b is deposited onto self cells, too.49 Therefore, all cells in contact with plasma without proper defenses are at risk of destruction, making continuous and efficient downregulation of complement essential to self cells. If the regulation on host cells is compromised, as is often seen in aHUS and occasionally in other forms of HUS, the amplified attack may rapidly cause cell and tissue damage.
Regulation of complement
The main complement regulators are the plasma protein factors H and I and the membrane proteins CD35, CD46, CD55, and CD59.50 Four of the control proteins, factor H, CD35, CD46, and CD55, control C3b on cell surfaces by acting as cofactors for factor I in proteolytic inactivation of C3b, competing with factor B in binding to C3b, accelerating decay of the formed C3bBb enzyme, or by a mixture of these effects.51 These proteins all contribute to the protection of endothelial cells; red blood cells lack CD4652 and platelets lack CD35.53,54 CD59 acts later in the cascade by blocking assembly of the lytic membrane attack complex on all plasma-exposed cells.
Studies on the pathogenesis of aHUS have revealed a central role of factor H in discriminating between self cells and microbes (ie, whether a target should be spared or destroyed).55,56 If factor H binds to C3b deposited onto a surface, activation is efficiently inhibited. In the absence of factor H binding, activation proceeds (Figure 2B).57 Simultaneous binding of factor H to C3b and cell surface molecules such as sialic acid or glycosaminoglycans is essential for efficient regulation of complement activation.55,58,59
Complement may cause cell damage in HUS
Mutations in complement proteins in aHUS
In STEC-HUS and secondary HUS, the disease process is usually initiated by phenomena distinct from the complement system or linked only indirectly to complement activation. In aHUS, however, complement is centrally involved. In aHUS, mutations have been reported in 5 central complement proteins. Most of these mutations lead to impaired regulation of alternative pathway activation. Some disturb recognition of C3b by factor H,56,60 factor I,61 or CD4662 ; some disturb recognition of self-cell surface molecules, such as sialic acid or glycosaminoglycans, by factor H.55,59 Some mutations in C3 or factor B prolong the C3 convertase half-life or prevent its elimination.63,64 The frequencies of mutations observed in aHUS are shown in Table 1. In addition, autoantibodies against factor H can interfere with regulation in a manner similar to mutations.69 The common feature for the mutations and autoantibodies is impaired control of the complement alternative pathway on self-cell surfaces.
Frequencies of the most common mutations identified in aHUS patients
| Mutated gene/protein . | Type . | Frequency (%)* . | Death or end-stage renal disease 3-10 y after onset (%)† . |
|---|---|---|---|
| Factor H (including CFH/CFHR1 hybrid genes) | Loss of complement regulation | 24-28 | 70-80 |
| MCP (CD46) | Loss of complement regulation | 5-9‡ | <20 |
| Factor I | Loss of complement regulation | 4-8 | 60-70 |
| C3 | Gain of complement activation | 2-8 | 60-70 |
| Factor B | Gain of complement activation | 0-4 | 70 |
| Thrombomodulin | Possibly loss of complement regulation and procoagulative state | 0-5 | 50-60 |
| CFHR1/3 deficiency with anti-factor H autoantibodies | Loss of complement regulation | 3-10§ | 30-70 |
| Diacylglycerol kinase ε | Prothrombotic | 0-3|| | 46 |
| None identified | 30-48 | 50 |
| Mutated gene/protein . | Type . | Frequency (%)* . | Death or end-stage renal disease 3-10 y after onset (%)† . |
|---|---|---|---|
| Factor H (including CFH/CFHR1 hybrid genes) | Loss of complement regulation | 24-28 | 70-80 |
| MCP (CD46) | Loss of complement regulation | 5-9‡ | <20 |
| Factor I | Loss of complement regulation | 4-8 | 60-70 |
| C3 | Gain of complement activation | 2-8 | 60-70 |
| Factor B | Gain of complement activation | 0-4 | 70 |
| Thrombomodulin | Possibly loss of complement regulation and procoagulative state | 0-5 | 50-60 |
| CFHR1/3 deficiency with anti-factor H autoantibodies | Loss of complement regulation | 3-10§ | 30-70 |
| Diacylglycerol kinase ε | Prothrombotic | 0-3|| | 46 |
| None identified | 30-48 | 50 |
Frequencies of the genetic abnormalities have been adopted from a recent review8 and cohort studies.65,66
Frequency of the isolated heterozygous MCP (CD46) mutation is usually 7% to 8%, but the mutations are frequently found in combination with other mutations in complement genes (up to 22%).67
Autoantibodies against factor H have been reported in 56% of pediatric aHUS cases in India.68
Diacylglycerol kinase ε mutations are most frequently found in patients with disease manifestation within the first year of life (5%-27% in this population).
Complement-mediated red cell and endothelial damage in HUS
Cells that are continuously exposed to complement alternative pathway activation include all cells in contact with plasma: red cells, platelets, leukocytes, and endothelial cells. The most vulnerable to rapid lysis seem to be red cells, because they lack an efficient membrane repair system as observed in nucleated cells. However, some shedding of the complement membrane attack complexes on these cells can occur,70 but contribution of this phenomenon to the protection of red cells from complement lysis has not been proven. Complement attack against red cells can rapidly lead to release of hemoglobin, as seen in paroxysmal nocturnal hemoglobinuria (PNH).71
There are two main mechanistic explanations underlying hemolysis and the appearance of red cell fragments and schistocytes in the peripheral blood in aHUS (ie, signs of microangiopathic hemolysis characteristic for TMAs).72,73 The first and traditional explanation is mechanical hemolysis as a result of narrowed microvasculature caused by microthrombi. However, narrowed capillaries have not been experimentally proven to lead to hemolysis or schistocytes in HUS. One of the main problems with this explanation is that deformability of normal red cells is high, and channels narrower than 3 µm in diameter do not allow passage of red cells at all.74 The second explanation, complement-mediated lysis, is also possible either as a primary or secondary cause, but the main challenge in this explanation is that red cell fragments and schistocytes are not typical for complement-mediated lysis in PNH.75 Thus, there may be alternative mechanisms linked to stiffening of the red cell membrane by complement C3d (or C3b) deposits.76,77 Further studies are needed to clarify the mechanism for the clinically characteristic hemolysis in HUS cases associated with complement regulation defects.
Endothelial cells and leukocytes can remove complement deposits and membrane attack complexes more efficiently than red cells, either by internalization or by shedding from the plasma membrane in microvesicles.78,79 In addition, these cells may also repair membrane damage. Endothelial cell damage is evident in histologic analysis of biopsies taken from HUS patients during the acute stage.80 The reason for endothelial cell damage in STEC-HUS is linked to Shiga toxin, whereas damage in aHUS has been attributed to lytic or sublytic complement attack.60,81 However, several other mechanisms affecting endothelial cells can cause or contribute to the damage. Examples of such factors are cell exposure to nitric oxide, free hemoglobin, activated neutrophils and monocytes or released reactive oxygen species from these cells, C3a or C5a, proinflammatory cytokines, proinflammatory or procoagulant microparticles, or cellular hypoxia due to microthrombi.
Cell damage–induced complement activation in HUS
Independent of the reason for the damage, injured endothelium may activate the complement cascade.82 This activation may occur not only via the alternative pathway but also (as observed in ischemia reperfusion injury) by the classical and lectin pathways via exposure of novel epitopes recognized by these pathways.83 In aHUS, the alternative pathway is likely to be responsible for the damage, because the disease is associated with dysregulation of this pathway in particular.8 The damage-induced enhancement of complement activation can lead to further damage of nearby cells, both the cells residing in the immediate area and those passing by in the vicinity.84 It has long been assumed that damage to endothelial cells is the first step in STEC-HUS and also in aHUS85,86 ; however, it is not known which cells are damaged first. Complement activation is also enhanced by activation of platelets87 and hemoglobin (and heme) release from red cells.88 Accordingly, in aHUS, simultaneous or even earlier damage of these cells compared with that of endothelial cells is possible although not proven.59
Platelets and coagulation in the pathogenesis of HUS
Platelets in the pathogenesis of aHUS
Complement-mediated platelet activation has gained much less attention in aHUS than hemolysis or endothelial cell damage. Platelets may, however, be involved, either primarily or secondarily, in pathogenesis of this disease because thrombocytopenia is a typical feature of TMAs,89 and the thrombi in capillaries and arterioles contain platelets90 in addition to red cells.91 Platelets are easily activated by complement attack92 via the formation of membrane attack complex on the platelet membrane,93 but some internalization or shedding of the complement membrane attack complexes can occur.40 Although platelets are normally protected from complement attack by the concerted action of factor H and membrane regulators,94 this protection is impaired in aHUS.20 We have recently shown that protection of platelets from complement attack by factor H requires sialic acids on the cell membrane in vitro.59 Therefore, impaired alternative pathway regulation is a logical explanation for thrombocytopenia and could also contribute to formation of platelet-rich microvascular thrombi.72,73
Links between complement, coagulation, and platelets in HUS
The common conclusion from the numerous studies linking complement and coagulation is that complement activation leads to initiation or enhancement of coagulation. Complement can trigger coagulation by 2 primary means. First, complement causes tissue damage via formation of membrane attack complexes on endothelial cells. Second, complement activation leads to release of C5a or formation of soluble C5b-9 complexes that can induce endothelial cell activation and expression of procoagulative tissue factor.95-97 In addition, numerous molecular interactions between complement and coagulation proteins have been described.98,99 The physiological relevance of most of these interactions has not been proven. Some interactions, such as cleavage of C5 by thrombin, may be clinically relevant.100,101
There are also links between complement and platelets. The most important of these seems to be the activation of platelets by membrane attack complexes92 or C5a.102 The procoagulative effect of membrane attack complexes seems to be mediated by platelet prothrombinase,103 not by irreversible cell damage.104 Independent of the reason for the activation of platelets, it is well known that their activation and aggregation is closely linked to coagulation, thereby creating another bridge between activation of the complement and coagulation cascades. In the pathogenesis of all 3 forms of HUS, formation of microthrombi in capillaries and arterioles is central. The most important links between complement activation, coagulation, and platelets in HUS are shown in Figure 3.
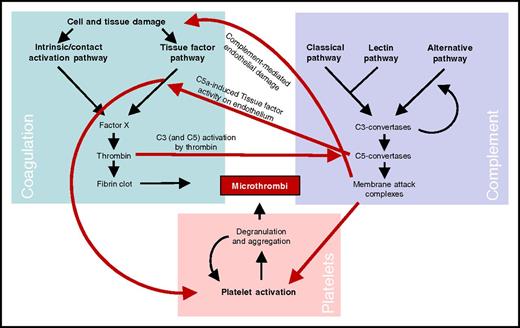
Schematic presentation of the main links between the complement and coagulation systems and platelets in formation of microthrombi in aHUS. Complement activation leads to release of the C5a peptide, inducing tissue factor activity on endothelial cells leading to a procoagulative state of the endothelium. Activation of the coagulation cascade leads to generation of active thrombin that is able to cleave not only fibrinogen but also complement C5, which thereby enables coagulation-enhanced complement activation. Formation of membrane attack complexes on endothelial cells and platelets can cause endothelial cell damage and platelet activation. Finally, activation of the coagulation system leads to platelet activation via various mechanisms. Together, the coagulation system and platelet activation/aggregation lead to formation of microthrombi. The importance of complement in this process in aHUS is clearly demonstrated by rapid inhibition of microvascular thrombosis by therapeutic complement inhibition.
Schematic presentation of the main links between the complement and coagulation systems and platelets in formation of microthrombi in aHUS. Complement activation leads to release of the C5a peptide, inducing tissue factor activity on endothelial cells leading to a procoagulative state of the endothelium. Activation of the coagulation cascade leads to generation of active thrombin that is able to cleave not only fibrinogen but also complement C5, which thereby enables coagulation-enhanced complement activation. Formation of membrane attack complexes on endothelial cells and platelets can cause endothelial cell damage and platelet activation. Finally, activation of the coagulation system leads to platelet activation via various mechanisms. Together, the coagulation system and platelet activation/aggregation lead to formation of microthrombi. The importance of complement in this process in aHUS is clearly demonstrated by rapid inhibition of microvascular thrombosis by therapeutic complement inhibition.
Complement in disease processes and therapy of HUS
Infections and complement activation
There are several reasons why infections are associated with HUS in general: direct cell or tissue damage caused by a microbe, as seen in STEC-HUS; indirect damage caused by complement activation promoted by the pathogens105 ; infection-associated autoantibody formation against factor H106 ; or possibly coagulation disorders during infection.24 The acute phase reaction may also contribute, because several complement proteins (at least C3, C1s, factor B, C5, C4, and C1q107,108 ) are acute phase reactants. This provides an explanation for the observed increase in complement hemolytic activity during the acute phase.109 Increased plasma concentrations of these proteins do not trigger complement activation as such but could promote activation upon complement-activating circumstances caused by the microbe itself or damaged host tissues.
Our recent study provided a novel possible explanation for why certain infections can lead to secondary HUS. We observed that sialic acid in in vitro assays is the main ligand for factor H on red cells and also on platelets and endothelial cells.59 Pneumococci23 and influenza A virus,24 most often seen in secondary HUS associated with infections, possess very active neuraminidases specialized in removing sialic acid from self cell surfaces.110,111 It is therefore possible that microbial enzymes could make self blood cells more vulnerable to the complement alternative pathway.112
Continuation of disease after removing the trigger
The course of STEC-HUS is usually self-limiting, although acute kidney injury and systemic involvement can complicate and prolong the disease. On the contrary, in some patients with aHUS, or sometimes secondary HUS, the acute disease continues after the trigger has been removed. This is especially striking in patients who have a mutation in a complement regulatory molecule for decades without symptoms but fail to control the disease even several months after it has been triggered. All the provided explanations for this phenomenon are controversial. One explanation is that complement attack against self cells during the acute disease flare leads to damage-induced amplification of 1 or more of the pathways, providing potential for longstanding complement activation. This is demonstrated by slow normalization of early complement activation markers such as C3 fragments or factor B only after several months of C5-blocking eculizumab therapy.113 Most patients do not experience a relapse upon discontinuation of therapeutic complement inhibition, although patients with factor H mutations often do.114,115 Another explanation for the longstanding disease process is that endothelial cell activation continues for months after the rapid termination of hemolysis and increase in platelet count.116
Therapeutic complement inhibition provides insight into pathogenesis of HUS
The impact of complement activation on the pathogenesis of STEC-HUS, aHUS, and secondary HUS seems to be variable, apparently because of the biological phenomena linked to the pathogenesis. The impact of complement is seen most clearly in observing the effects of therapeutic complement inhibition on these diseases. As expected, complement inhibition is effective in patients who have a mutation in complement molecules or autoantibodies against factor H. The efficacy of therapeutic complement inhibition in patients with secondary HUS has not been systematically assessed, but it has been shown to be beneficial in a number of patients who do not have a mutation in any of the studied complement proteins.117 These include patients with autoimmunity (eg, scleroderma),118 systemic lupus erythematosus,119,120 or catastrophic antiphospholipid syndrome121,122 and secondary HUS associated with postpartum period,123 HIV infection,124 use of cytotoxic drugs,35 and either bone marrow or solid organ transplantation.27,28 On the basis of these multiple single cases, it seems that complement is involved in at least some patients with secondary HUS. There is currently insufficient evidence to classify secondary HUS into complement-driven and complement-independent forms, but this might become a clinically relevant subclassification of secondary HUS in the future, as hinted at by some diagnostic algorithms.15,125 From a clinical point of view, it already is important and will probably be even more important in the future for making justified decisions regarding which secondary HUS patients will need expensive therapeutic complement inhibitors.
Although complement clearly contributes to disease pathogenesis in some patients with STEC-HUS,126 and some patients seem to benefit from early administration of eculizumab,127 most STEC-HUS patients do not respond clearly to this drug.21 TTP is also not generally considered to be an indication for eculizumab treatment,128,129 mainly because plasma therapy is usually effective. Despite this, eculizumab has been beneficial in some TTP cases that are refractory to conventional therapy.130,131 Eculizumab does not seem to be effective for the aHUS patients in whom a DGKε mutation is found10,113,132 or in those with cobalamin C deficiency in adulthood133 ; however, this treatment has been attempted in only a limited number of patients.
Multiple parallel processes leading to clinically significant tissue damage
Parallel processes in pathogenesis of aHUS
The cellular or molecular consequences discussed above can be summarized as parallel or consecutive phenomena with regard to the various cells involved and the clinical signs typical for HUS and aHUS, thereby forming a model of HUS pathogenesis (Figure 4). In this model, the process is not linear but cyclical, and possibly all the processes happen simultaneously in vivo. However, such a process may be initiated from different stages, resulting in a similar vicious cycle. The primary point of process initiation could be in complement-mediated cell damage, direct activation of platelets, initiation of a procoagulative state in the endothelium, dysregulation of the coagulation or thrombolytic pathways, or direct complement-independent cell damage.
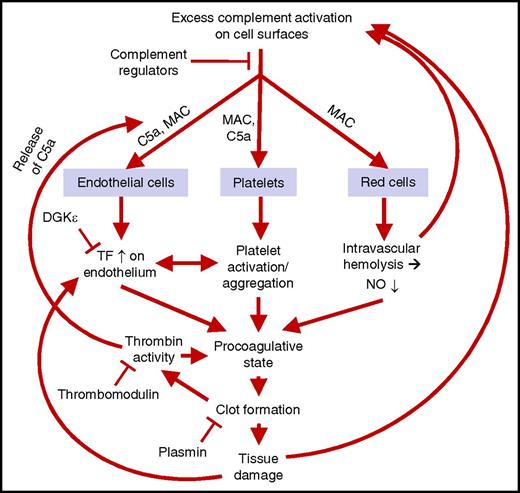
A model of the parallel pathogenic processes in HUS. Excess complement activation on endothelial cell, platelet, and red cell surfaces leads to C5a release and membrane attack complex (MAC) formation. This leads to enhanced tissue factor (TF) activity on the endothelium, activation and aggregation of platelets, and release of hemoglobin and reduction of nitric oxide (NO) in plasma. These phenomena lead to a procoagulative state, coagulation, and thrombosis-mediated tissue damage. There are several feedback loops in this process. These loops can be seen as a cycle that may be initiated at several points and where several phenomena may take place in parallel. This may explain why STEC-HUS, secondary HUS, and aHUS share clinical features although the processes start in various ways. DGKε, diacylglycerol kinase ε.
A model of the parallel pathogenic processes in HUS. Excess complement activation on endothelial cell, platelet, and red cell surfaces leads to C5a release and membrane attack complex (MAC) formation. This leads to enhanced tissue factor (TF) activity on the endothelium, activation and aggregation of platelets, and release of hemoglobin and reduction of nitric oxide (NO) in plasma. These phenomena lead to a procoagulative state, coagulation, and thrombosis-mediated tissue damage. There are several feedback loops in this process. These loops can be seen as a cycle that may be initiated at several points and where several phenomena may take place in parallel. This may explain why STEC-HUS, secondary HUS, and aHUS share clinical features although the processes start in various ways. DGKε, diacylglycerol kinase ε.
Interplay between complement and thrombosis in other thrombotic diseases
HUS and aHUS are not the only diseases in which thrombosis and imbalance between complement activation and regulation are seen. Accordingly, it may be relevant for clinical hematologists to have insight into the role of complement in these diseases as well. In some of these diseases, such as PNH,134 sepsis syndrome,135 disseminated intravascular coagulation,3 or ischemia-reperfusion injury,84 the alternative pathway contributes to or is even fully responsible for initiating the complement activation. However, the pathologic defect leading to these diseases may be independent of the alternative pathway. For example, in PNH, the alternative pathway activation is responsible for the physiological challenge of blood cells with C3b; this leads to cell damage only because of the absence of glycophosphoinositol-anchored complement regulators CD55 and CD59.134 In some others, such as catastrophic antiphospholipid antibody syndrome, antibody-mediated rejection, or scleroderma renal crisis, the classical pathway is putatively mostly responsible for complement overactivation, although the other complement pathways may also contribute.
The common feature for the clinically different thrombotic diseases with complement activation could be a vicious cycle. Such a cycle could be formed between complement-mediated cell damage (to endothelial cells, platelets, and red cells), formation of fibrin or platelet thrombi (or both), tissue hypoxia as a result of vascular occlusion, further tissue damage, and damage-induced secondary complement overactivation (Figure 5). Most of the links in this kind of self-amplifying process are evident, but the clinical importance of some others in human diseases remains to be shown. The use of therapeutic complement inhibition beyond the current indications of eculizumab (aHUS and PNH) might demonstrate the relevance of complement in at least some of these disease processes.
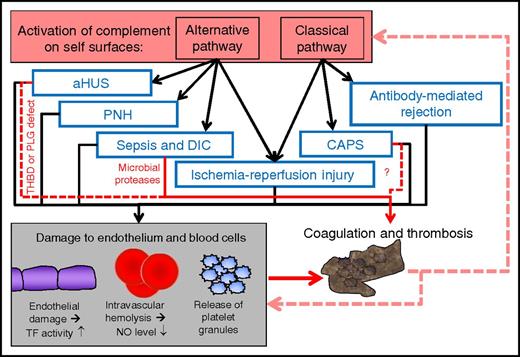
Schematic model of the role of complement activation, cell damage, and thrombosis in various severe diseases or conditions with major thrombotic problems. Activation of complement can occur via the alternative pathway (in the absence of antibodies) or the classical pathway (in the presence of target-bound antibodies). In each of the indicated diseases or conditions, endothelium, red cells, or platelets are damaged. This may contribute to coagulation and thrombosis, and direct procoagulative effects may also participate (red arrows). Thrombosis can lead to further tissue damage and increasing complement activation. The process can enhance itself via positive feedback loops and form a vicious cycle. Although complement is involved in each of these diseases, its impact needs to be clarified in clinical studies. CAPS, catastrophic antiphospholipid syndrome, DIC, disseminated intravascular coagulation; PLG, plasminogen; THBD, thrombomodulin.
Schematic model of the role of complement activation, cell damage, and thrombosis in various severe diseases or conditions with major thrombotic problems. Activation of complement can occur via the alternative pathway (in the absence of antibodies) or the classical pathway (in the presence of target-bound antibodies). In each of the indicated diseases or conditions, endothelium, red cells, or platelets are damaged. This may contribute to coagulation and thrombosis, and direct procoagulative effects may also participate (red arrows). Thrombosis can lead to further tissue damage and increasing complement activation. The process can enhance itself via positive feedback loops and form a vicious cycle. Although complement is involved in each of these diseases, its impact needs to be clarified in clinical studies. CAPS, catastrophic antiphospholipid syndrome, DIC, disseminated intravascular coagulation; PLG, plasminogen; THBD, thrombomodulin.
Conclusions
Complement activation is central in the pathogenesis of aHUS. Complement activation may also contribute to pathogenesis in some patients with STEC-HUS and secondary HUS, either by regulation defects or by triggers that lead to enhanced complement activation. STEC-HUS is associated with a gastrointestinal infection with STEC, and secondary HUS is associated with a coexisting disease, whereas aHUS is usually associated with complement dysregulation leading to attack against all cells in contact with plasma. The typical clinical triad of microangiopathic hemolytic anemia, thrombocytopenia, and organ damage can be explained in STEC-HUS by the cytotoxic effects of Shiga toxin and in secondary HUS and aHUS usually by complement-mediated processes.
It is logical that therapeutic complement inhibition is effective in aHUS. The impact of complement in the pathogenesis of STEC-HUS and secondary HUS is still uncertain. Nevertheless, at least some patients seem to benefit from therapeutic complement inhibition, indicating that complement may also be involved in these diseases.
In the pathogenesis of aHUS, STEC-HUS, and at least some forms of secondary HUS, the interplay between complement, coagulation, and platelets seems to be essential. The primary point of process initiation could be either complement-mediated or not. Independent of the initiation point, the process may have similar features because of numerous feedback loops and powerful amplification under certain conditions. In general, it is likely that HUS and aHUS are not the only diseases in which the feedback loop among cell damage, coagulation and thrombosis, hypoxia-mediated endothelial activation, and activation of complement form a vicious cycle. Therefore, therapeutic complement inhibition may prove to be beneficial for a number of systemic thrombotic diseases in the future.
Acknowledgments
The author acknowledges Derek Ho, a postdoctoral fellow in the Jokiranta Research Group at the University of Helsinki, for proofreading the manuscript and Satu Hyvärinen for fruitful discussions on the pathogenesis of atypical hemolytic uremic syndrome.
Conflict-of-interest disclosure: T.S.J. has received lecture honoraria or consultation fees from AbbVie, Alexion Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly, Medac Pharma, Merck Sharp & Dohme, Pfizer, Roche, and Takeda Pharmaceuticals.
Correspondence: T. Sakari Jokiranta, Research Programs Unit, Immunobiology, Haartmaninkatu 3, FIN-00014, University of Helsinki, Helsinki, Finland; e-mail: sakari.jokiranta@helsinki.fi.

![Figure 2. Consequences of complement activation. (A) The complement system can be activated via 3 pathways: classical, lectin, and alternative. All pathways lead to formation of powerful enzymes, the C3-convertases, followed by activation of the terminal cascade. The main effector functions of complement (promotion of opsonophagocytosis by opsonization and chemotaxis and formation of lytic membrane attack complexes) aim to destroy harmful agents such as microbes. Lysis of target cells can lead to damage-induced enhancement of complement activation. (B) Alternative pathway activation is based on continuous, low-level covalent deposition of C3b molecules onto practically all surfaces in contact with plasma. If the C3b molecule is allowed to form an enzyme (shown in red), new C3b deposits will be formed around the enzyme leading to rapid amplification of the activation. If the regulator factor H binds to C3b, the convertase enzyme is inactivated and no complement activation follows. The simultaneous interaction of factor H with both C3b and cell surface sialic acids (or possibly glycosaminoglycans [GAGs]) is essential for proper regulation on self red cells, platelets, and endothelial cells. If this fails, disbalance between activation and regulation may lead to pathogenesis of atypical HUS. iC3b, C3b molecule incapable of forming an enzyme with factor B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/21/10.1182_blood-2016-11-709865/4/m_blood709865f2.jpeg?Expires=1771007333&Signature=VgQOZouml~hS9Cl5rY3E8iYv2grYd0~Ci91vGvi7oJjoSxg08fBD9fKq5WLE54eX~FT9Uhf25Yk-xz1GRrmGcwpw08txSMwVHwrvYiN4Z53~lO1aT5j8CPyo9uTVoJFK8wtiZoC1uN5h5qgjLKdP9djGtgpkPhEfkJgN9VG7eDFmq~dKguL08-KxI5vFyht09oyARKodg81TwAvGMmVi0~k7OfRsq7pX6JVmoOUgR0dym0R8xjkUtVAxfnqRREN13ShQK6FJIc0eq~Ul9ovKhF77iwzrtyoEgCvcE7koE0Znfl3ochjh3tMhyTuhIixCWQP7t~mO1GDF1CjcQ2VpRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal