Key Points
Factor XI deficiency is associated with reduced risk of cardiovascular events.
Factor XI deficiency is associated with reduced risk of VTE.
Abstract
Factor XI deficiency is one of the rare inherited coagulation factor deficiencies. However, its incidence is high within the Ashkenazi Jewish community. Because factor XI displays both procoagulant and antifibrinolytic activities, it has been postulated that an underlying cardiovascular benefit may exist with factor XI deficiency. This historical cohort study was performed using the electronic database of Clalit Health Services, the largest health care provider in Israel. All adults tested for factor XI activity between 2002 and 2014 were included in the study. Factor XI activity was classified into 3 categories: normal (activity >50%), mild deficiency (activity = 30%-50%), and moderate–severe deficiency (activity ≤30%). The cohort was followed until 31 December 2015 for incidence of cardiovascular events (composite of myocardial infarction, stroke, and transient ischemic attack) and venous thromboembolism (VTE). Of the 10 193 included patients, 8958 (88.9%) had normal factor XI activity, 690 (6.8%) had mild deficiency, and 542 (5.3%) had moderate–severe deficiency. Compared with individuals with normal activity, the adjusted hazard ratio (HR) for cardiovascular events was 0.52 (95% confidence interval [CI], 0.31-0.87) in those with mild deficiency, and 0.57 (95% CI, 0.35-0.93) in those with moderate–severe factor XI deficiency. The incidence of VTE was lower in those with factor XI deficiency (activity <50%) compared with those with normal activity; adjusted HR = 0.26 (95% CI, 0.08-0.84). In summary, factor XI deficiency is associated with decreased incidence of cardiovascular events and VTE.
Introduction
Israel contains within it a unique variety of groups predisposed to autosomal recessive diseases secondary to the history of segregated religious communities and relatively high likelihood to intermarry.1 Such groups attract research on autosomal recessive diseases and the generation of data with the results ideally spurring recognition and treatment of these conditions. Indicated groups include Ashkenazi Jews and Israeli Arabs.2,3 Severe factor XI deficiency, also known as hemophilia C, is reported to have prevalence of 1:450 within the Ashkenazi Jewish community as compared with a 1:106 worldwide.4 More modest factor XI deficiency, some of which may be autosomal dominant, has been estimated to be more common at 1 in 10 000 to 50 000.5 The estimated risk of heterozygosity for factor XI deficiency in Ashkenazi Jews can be as high as 9% and 3.3% in Iraqi Jews.6
Factor XI is part of the coagulation cascade and functions, when activated as part of the intrinsic pathway, to activate factor IX.7 Factor XI is also essential in the propagation of the coagulation cascade and thrombin generation, as well as in the downregulation of fibrinolysis.8,9 It is possible and rather common for factor XI deficiency to remain masked for the greater portion of one’s life without evident clinical symptoms as there is a poor correlation between factor XI deficiency and bleeding tendency.10 Because of its unique features, the deficiency can emerge as unexpected bleeding with an elongated activated partial thromboplastin time (APTT) during a surgery or on routine blood testing in an asymptomatic individual with no bleeding history.4 Occasionally it can present earlier on in life during a circumcision or at menarche with increased bleeding. The usual bleeding sites include areas with high fibrinolytic activity such as the nasopharynx and the genitourinary tract.11
Because factor XI displays both procoagulant and antifibrinolytic activities, it has been postulated that an underlying cardiovascular benefit may protect factor XI–deficient subjects. Small studies showed that severe factor XI deficiency is associated with reduced incidences of deep vein thrombosis12 and ischemic stroke,13 but not with myocardial infarction (MI).14
We hypothesized that factor XI deficiency is associated with reduced arterial and venous thromboembolic events. We presumed that the Clalit Health Services (CHS) database, which is a population-based database, will provide a suitable platform to help clarify this issue.
Materials and methods
Data source
CHS is a not-for-profit health care provider covering more than half of the Israeli population.15,16 The electronic medical record database of CHS includes data from multiple sources: primary care physicians, specialty clinics within the community, hospitalizations, laboratories, and pharmacies. A chronic disease registry is being compiled from these data sources. Diagnoses are captured in the registry by diagnosis-specific algorithms, employing International Classification of Diseases Ninth revision (ICD-9) code reading, text reading, laboratory test results, and disease-specific drug usage. A record is kept of the sources and dates used to establish the diagnosis, with the earliest recorded date considered to be the date of the diagnosis.
Selection of study population
CHS laboratory computerized database was retrospectively searched for all factor XI activity tests performed in adult individuals age 18 years or older between 1 January 2002 and 31 December 2014. We found 11 429 factor XI activity tests performed during this period. The lowest test result was used in individuals who had >1 factor XI activity test. Overall, 10 193 adult individuals were included in this study. The study was approved by a centralized institutional review board committee.
Definition of outcomes and follow-up
The association of factor XI deficiency was assessed with 2 outcomes: (1) venous thromboembolism (VTE) and (2) cardiovascular events that include MI, stroke, and transient ischemic attack (TIA). Individuals were followed from the date of factor XI activity test until the outcome event, death, loss to follow-up, or 31 December 2015, whichever came first.
Study variables
In addition to the factor XI activity test results, the following data were retrieved from the computerized database of the CHS: demographic variables, presence of risk factors, selected chronic medical conditions at baseline, and antithrombotic medications use.
Demographic variables included age and sex. Comorbid conditions and risk factors included hypertension, diabetes mellitus, hyperlipidemia, smoking, congestive heart failure (CHF), and history of stroke, ischemic heart disease (IHD), atrial fibrillation, chronic obstructive pulmonary disease (COPD), liver cirrhosis, and malignancy. Medication use was defined as at least 1 prescription filled in the 90 days prior to study entry. Antithrombotic medication use was assessed for each antiplatelet and anticoagulant drug (ie, vitamin K antagonists, direct thrombin inhibitors, direct factor Xa inhibitors).
Factor XI activity assay
The factor XI assay is performed using a modified APTT assay and a factor-deficient plasma substrate. Subject plasma is combined and incubated with a factor XI–deficient plasma substrate and an APTT reagent. After a specified incubation time, calcium is added to trigger the coagulation process in the mixture. At this time, the time to clot formation is measured optically, which correlates with factor level in the subject plasma.17
Statistical methods
Continuous variables were summarized with mean ± standard deviation, and categorical variables were presented as numbers and proportions. Factor XI activity was classified into 3 predefined categories: (1) factor XI activity ≤30% (moderate–severe deficiency), (2) factor XI activity 30% to 50% (mild deficiency), and (3) factor XI activity >50% (normal activity). Comparisons of baseline characteristics between the 3 categories of factor XI activity were performed using the χ2 test for categorical variables and using analysis of variance for continuous variable (P value of the global test is reported). Post hoc comparisons between groups were performed using Bonferroni correction. The annualized incidence rates of cardiovascular events and VTE were estimated by dividing the number of incident cases of these outcomes by the total follow-up time and were expressed as number per 1000 person-years of observation. Age-adjusted survival function curves were used to plot the distribution of time to reach cardiovascular events and VTE by factor XI activity category. Cox proportional hazard regression models were used to assess the association between factor XI activity and the study outcomes and to estimate the crude and the adjusted hazard ratios (HRs) along with 95% confidence interval (95% CI) using normal factor XI activity (>50%) as the reference category. As factor XI deficiency is present at birth, we performed an additional analysis using left truncation method in which follow-up began from birth, and age at the time of factor XI activity test was included as a covariate along with the other confounders.
In addition, we performed the following sensitivity analyses: (1) restricting the analysis to women only; (2) using different cutoffs for the classification of factor XI categories (factor XI activity ≤15% [severe deficiency], factor XI activity 15% to 50% [mild–moderate deficiency], and normal activity >50%]; and (3) performing additional analyses because some individuals with normal factor XI activity have elongated APTT that could have been caused by conditions that might be associated with the study outcomes, such as lupus anticoagulant (first, excluding all those individuals with elongated APTT from the group with normal factor XI activity and, second, excluding only those with elongated APTT and lupus anticoagulant).
All statistical analyses were performed using IBM SPSS Statistics 23.0 (IBM, New York, NY). For all analyses, P < .05 for the 2-tailed tests was considered to be statistically significant.
Results
The study included 10 193 adults. The mean age was 41.0 ± 37.5 years and median age 35.5 (interquartile range [IQR], 28.5-51.6) years. Of them 7921 (77.7%) were females with mean age of 37.7 ± 14.6 years and median age of 33.5 (IQR, 27.8-42.7) years. Factor XI activity ≤30% (moderate–severe deficiency) was measured in 542 (5.3%), factor XI activity 30% to 50% (mild factor XI deficiency) in 690 (6.8%), and factor XI activity >50% (normal activity) in 8958 (88.9%) of included individuals (Table 1).
Demographic and clinical baseline characteristics of study participants (n=10 193)
| Variable . | Total (n = 10 193) . | Categories of factor XI deficiency . | P . | ||
|---|---|---|---|---|---|
| Moderate–severe [≤30%] (n = 542) . | Mild [30%-50%] (n = 693) . | Normal [>50%] (n = 8958) . | |||
| Age, y | 41.0 ± 35.5 | 54.5 ± 21.1 | 47.0 ± 21.0 | 39.7 ± 15.6 | <.001*†‡ |
| Male sex | 2272 (22.3%) | 230 (42.4%) | 293 (42.3%) | 1749 (19.5%) | <.001*† |
| Anticoagulants | 830 (8.1%) | 29 (5.4%) | 50 (7.2%) | 751 (8.4%) | .028* |
| Antiplatelet | 1752 (17.2%) | 121 (22.3%) | 128 (18.5%) | 1503 (16.8%) | .003* |
| Atrial fibrillation | 302 (3.0%) | 44 (8.1%) | 69 (10%) | 189 (2.1%) | <.001*† |
| Malignancy | 762 (7.5%) | 76 (14.0%) | 84 (12.1%) | 602 (6.7%) | <.001*† |
| Diabetes | 651 (6.4%) | 67 (12.4%) | 72 (10.4%) | 512 (5.7%) | <.001*† |
| Cirrhosis | 104 (1.0%) | 23 (4.2%) | 32 (4.6%) | 49 (0.5%) | <.001*† |
| COPD | 179 (1.8%) | 17 (3.1%) | 20 (2.9%) | 142 (1.6%) | .002*† |
| Hypertension | 1642 (16.1%) | 199 (36.7%) | 204 (29.4%) | 1239 (13.8%) | <.001*†‡ |
| IHD | 238 (2.3%) | 21 (3.9%) | 32 (4.6%) | 185 (2.1%) | <.001*† |
| Stroke | 495 (4.9%) | 28 (5.2%) | 39 (5.6%) | 428 (4.8%) | .570 |
| CHF | 185 (1.8%) | 25 (4.6%) | 30 (4.3%) | 130 (1.5%) | <.001*† |
| Hyperlipidemia | 3952 (38.8%) | 278 (51.3%) | 271 (39.1%) | 3403 (38.0%) | <.001*‡ |
| Smoking | 2861 (28.1%) | 149 (27.5%) | 202 (29.1%) | 2510 (28.0%) | .779 |
| Variable . | Total (n = 10 193) . | Categories of factor XI deficiency . | P . | ||
|---|---|---|---|---|---|
| Moderate–severe [≤30%] (n = 542) . | Mild [30%-50%] (n = 693) . | Normal [>50%] (n = 8958) . | |||
| Age, y | 41.0 ± 35.5 | 54.5 ± 21.1 | 47.0 ± 21.0 | 39.7 ± 15.6 | <.001*†‡ |
| Male sex | 2272 (22.3%) | 230 (42.4%) | 293 (42.3%) | 1749 (19.5%) | <.001*† |
| Anticoagulants | 830 (8.1%) | 29 (5.4%) | 50 (7.2%) | 751 (8.4%) | .028* |
| Antiplatelet | 1752 (17.2%) | 121 (22.3%) | 128 (18.5%) | 1503 (16.8%) | .003* |
| Atrial fibrillation | 302 (3.0%) | 44 (8.1%) | 69 (10%) | 189 (2.1%) | <.001*† |
| Malignancy | 762 (7.5%) | 76 (14.0%) | 84 (12.1%) | 602 (6.7%) | <.001*† |
| Diabetes | 651 (6.4%) | 67 (12.4%) | 72 (10.4%) | 512 (5.7%) | <.001*† |
| Cirrhosis | 104 (1.0%) | 23 (4.2%) | 32 (4.6%) | 49 (0.5%) | <.001*† |
| COPD | 179 (1.8%) | 17 (3.1%) | 20 (2.9%) | 142 (1.6%) | .002*† |
| Hypertension | 1642 (16.1%) | 199 (36.7%) | 204 (29.4%) | 1239 (13.8%) | <.001*†‡ |
| IHD | 238 (2.3%) | 21 (3.9%) | 32 (4.6%) | 185 (2.1%) | <.001*† |
| Stroke | 495 (4.9%) | 28 (5.2%) | 39 (5.6%) | 428 (4.8%) | .570 |
| CHF | 185 (1.8%) | 25 (4.6%) | 30 (4.3%) | 130 (1.5%) | <.001*† |
| Hyperlipidemia | 3952 (38.8%) | 278 (51.3%) | 271 (39.1%) | 3403 (38.0%) | <.001*‡ |
| Smoking | 2861 (28.1%) | 149 (27.5%) | 202 (29.1%) | 2510 (28.0%) | .779 |
Significant difference after Bonferroni correction between moderate–severe and normal groups.
Significant difference after Bonferroni correction between mild and normal groups.
Significant difference after Bonferroni correction between moderate–severe and mild groups.
Individuals with normal factor XI activity were younger and were more likely to be females compared with individuals with mild and moderate–severe factor XI deficiency. Individuals with normal factor XI activity were less likely to have comorbidities compared with individuals with mild and moderate–severe factor XI deficiency. These comorbidities include hypertension, diabetes, CHF, IHD, COPD, liver cirrhosis, atrial fibrillation, malignancy, and history of stroke. This lower prevalence of comorbidities is likely explained by the younger average age of this group.
Patients with factor XI deficiency (factor XI activity ≤50%) were more likely to have prior history of gastrointestinal bleeding compared with patients with normal factor XI activity, 2.9% and 0.6%, respectively (P < .001). Prior history of intracerebral hemorrhage was observed in 3.3% of patients with factor XI deficiency (factor XI activity ≤50%) compared with 3.1% in patients with normal factor XI activity (P = .689). A prior history of menometrorrhagia was observed in 2.4% of patients with factor XI deficiency (factor XI activity ≤50%) compared with 5.3% in patients with normal factor XI activity (P < .001).
Of the 8958 individuals with normal factor XI activity, 965 (10.8%) had elongated APTT. Of them, 272 (28.2%) had at least 1 positive test result for lupus anticoagulants; 10.2%, factor IX deficiency; 9.4%, factor VIII deficiency; 9.3%, factor VII deficiency; 2.6%, factor X deficiency; and 2.2%, von Willebrand factor deficiency.
Association of factor XI deficiency and cardiovascular events
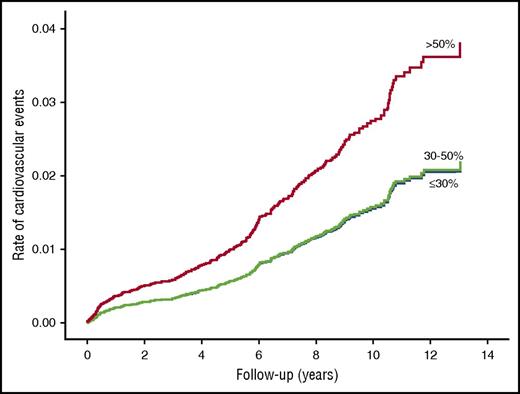
Cardiovascular events (MI/stroke/TIA) developed in 255 patients during 63 288 person-years of follow-up (incidence rate 4.03 per 1000 person-years). The age-adjusted incidence rate of cardiovascular events was lower in those with factor XI deficiency compared with those with normal factor XI activity (Figure 1; Table 2). The results were similar after further adjustment for sex, risk factors, comorbidities, and antithrombotic drug use. Compared with patients with normal factor XI activity, the HR for cardiovascular events was 0.57 (95% CI, 0.35-0.93) in patients with moderate–severe deficiency, and 0.52 (95% CI, 0.31-0.87) in patients with mild factor XI deficiency (Table 2). The results were similar when the analysis was performed with the left truncation method (Table 3).
Age-adjusted survival function curves of patients with normal factor XI activity (>50%), mild deficiency (30%-50%), and moderate–severe deficiency (≤30%) for future cardiovascular events.
Age-adjusted survival function curves of patients with normal factor XI activity (>50%), mild deficiency (30%-50%), and moderate–severe deficiency (≤30%) for future cardiovascular events.
Adjusted HRs for the association of factor XI activity with VTE and cardiovascular events (n=10 193)
| Study outcome . | Factor XI activity . | Number . | Events . | Age-adjusted model HR (95% CI) . | Fully adjusted model HR (95% CI) . |
|---|---|---|---|---|---|
| Cardiovascular event | ≤30% | 542 | 19 | 0.56 (0.35-0.91) | 0.57 (0.35-0.93) |
| 30%-50% | 693 | 16 | 0.57 (0.34-0.95) | 0.52 (0.31-0.87) | |
| >50% | 8958 | 230 | Reference | Reference | |
| VTE | ≤50% | 1235 | 3 | 0.14 (0.04-0.44) | 0.26 (0.08-0.84) |
| >50% | 8958 | 94 | Reference | Reference |
| Study outcome . | Factor XI activity . | Number . | Events . | Age-adjusted model HR (95% CI) . | Fully adjusted model HR (95% CI) . |
|---|---|---|---|---|---|
| Cardiovascular event | ≤30% | 542 | 19 | 0.56 (0.35-0.91) | 0.57 (0.35-0.93) |
| 30%-50% | 693 | 16 | 0.57 (0.34-0.95) | 0.52 (0.31-0.87) | |
| >50% | 8958 | 230 | Reference | Reference | |
| VTE | ≤50% | 1235 | 3 | 0.14 (0.04-0.44) | 0.26 (0.08-0.84) |
| >50% | 8958 | 94 | Reference | Reference |
Adjusted HRs using the left truncation method (n=10 193)
| Study outcome . | Factor XI activity . | Number . | Events . | Age-adjusted model HR (95% CI) . | Fully adjusted model HR (95% CI) . |
|---|---|---|---|---|---|
| Cardiovascular event | ≤30% | 542 | 19 | 0.58 (0.36-0.93) | 0.57 (0.35-0.92) |
| 30%-50% | 693 | 16 | 0.59 (0.35-0.98) | 0.51 (0.30-0.87) | |
| >50% | 8958 | 230 | Reference | Reference | |
| VTE | ≤50% | 1235 | 3 | 0.15 (0.05-0.47) | 0.26 (0.08-0.84) |
| >50% | 8958 | 94 | Reference | Reference |
| Study outcome . | Factor XI activity . | Number . | Events . | Age-adjusted model HR (95% CI) . | Fully adjusted model HR (95% CI) . |
|---|---|---|---|---|---|
| Cardiovascular event | ≤30% | 542 | 19 | 0.58 (0.36-0.93) | 0.57 (0.35-0.92) |
| 30%-50% | 693 | 16 | 0.59 (0.35-0.98) | 0.51 (0.30-0.87) | |
| >50% | 8958 | 230 | Reference | Reference | |
| VTE | ≤50% | 1235 | 3 | 0.15 (0.05-0.47) | 0.26 (0.08-0.84) |
| >50% | 8958 | 94 | Reference | Reference |
We reached similar results when patients with normal factor XI activity and elongated APTT were excluded from the analysis. Compared with patients with normal factor XI activity, the adjusted HR for cardiovascular events was 0.58 (95% CI, 0.35-0.95) in patients with moderate–severe deficiency, and 0.52 (95% CI, 0.31-0.89) in patients with mild factor XI deficiency. The results were consistent when we excluded from the normal group those with both elongated APTT and at least 1 positive test for lupus anticoagulant.
Restricting the analysis to women only demonstrated a slightly greater decrease in cardiovascular risk with mild factor XI deficiency (HR = 0.32; 95% CI, 0.12-0.91) than with moderate–severe deficiency (HR = 0.43; 95% CI, 0.20-0.96), suggesting a J-shaped relationship between factor XI deficiency and cardiovascular risk.
A J-shaped relationship is also suggested when a different classification categories of factor XI deficiency is used; compared with those with factor XI activity >50% (normal activity), the adjusted HR for cardiovascular events was 0.49 (95% CI, 0.30-0.80) in patients with factor XI activity of 15% to 50% (mild–moderate deficiency), and 0.63 (95% CI, 0.38-1.04) in patients with factor XI ≤15% (severe deficiency).
Association of factor XI deficiency and VTE
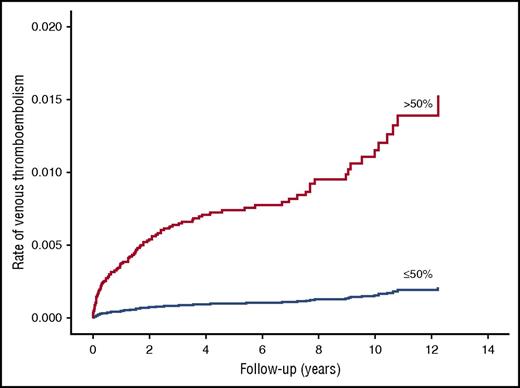
VTE developed in 97 patients during 63 750 person-years of follow-up (incidence rate 1.52 per 1000 person-years). None of the patients with factor XI activity ≤30% (moderate–severe deficiency) had a VTE during follow-up. Therefore, patients with moderate–severe deficiency were combined with patients with mild deficiency into 1 category (factor XI activity ≤50%) and compared with those with normal factor XI activity. The age-adjusted incidence rate of VTE was lower in those with factor XI deficiency compared with those with normal factor XI activity (Table 2; Figure 2). The fully adjusted HR for VTE was 0.26 (95% CI, 0.08-0.84) in patients with deficient factor XI (activity ≤50%) compared with those with normal activity (factor XI activity >50%). The results were similar when the left truncation method was used for the analysis (Table 3). A reduced risk of VTE was also demonstrated with factor XI activity of 30% to 50% (mild deficiency); adjusted HR was 0.39 (95% CI, 0.12-1.27) compared with factor XI activity >50% (normal activity). The observation that no patient with moderate to severe factor XI deficiency had a VTE event and the observed reduced risk of VTE with mild factor XI deficiency suggest a dose-response relationship between factor XI deficiency and VTE.
Age-adjusted survival function curves of patients with normal factor XI activity (>50%) and factor XI deficiency (≤50%) for future VTE events.
Age-adjusted survival function curves of patients with normal factor XI activity (>50%) and factor XI deficiency (≤50%) for future VTE events.
We reached similar results when patients with normal factor XI activity and elongated APTT were excluded from the analysis. Compared with patients with factor XI activity >50% (normal activity), the adjusted HR for VTE was 0.23 (95% CI, 0.07-0.76) in patients with factor XI activity ≤50%. The results were similar when we excluded from the normal group those with both elongated APTT and at least 1 positive test for lupus anticoagulant.
Only 1 VTE event was observed among the 712 women with factor XI activity <50%. Restricting the analysis to women only demonstrated a similar risk reduction of VTE; adjusted HR was 0.22 (95% CI, 0.03-1.63) in women with factor XI activity ≤50% compared with those with factor XI activity >50% (normal activity).
Discussion
This study demonstrates that factor XI deficiency is significantly associated with a decreased incidence of cardiovascular events (stroke, TIA, and MI) and a reduced risk of VTE. The results were consistent and robust with left truncation analysis and on sensitivity analysis. Although the magnitude of relative risk reduction in cardiovascular events was similar to those with mild and to those with moderate–severe factor XI deficiency, sensitivity analysis suggests a J-shaped relationship with the greatest cardiovascular benefit achieved with mild–moderate factor XI deficiency and not with severe deficiency. The relationship with VTE, however, seems to follow a dose-response pattern.
A previous small study that included 96 patients with severe factor XI deficiency showed that the observed incidence of MI was similar to the expected incidence in the general population.14 In a later publication, the same authors studied 115 patients with severe factor XI deficiency and detected that the observed incidence of ischemic stroke, but not of MI, was significantly lower than the expected incidence in the general population.13 Interestingly, a case-control study (all participants with factor XI activity ≥55%) demonstrated that the risk of MI increased with increasing factor XI activity even among subjects with normal factor XI activity.18 Supportive evidence from animal studies demonstrated significant inhibition in atherosclerosis in factor XI/apoE double knockout mice.19
The overall incidence of VTE was 1.52 per 1000 person-years. This incidence rate of VTE is higher than the previously reported incidence of 0.74 in the United Kingdom,20 and 1.0 per 1000 person-years reported in the United States.21 Yet, a study from France reported an incidence rate of VTE of 1.83 per 1000 person-years.22 The reduced risk of VTE associated with factor XI deficiency is in line with a previous study that observed not 1 case of DVT among 219 adults with severe factor XI deficiency, a finding that was significantly different from the expected number of DVTs from a population-based cohort.12 In our study, however, the observation that no patient with moderate to severe factor XI deficiency had a VTE event and the observed reduced risk of VTE with mild factor XI deficiency suggest a dose-response relationship between factor XI deficiency and VTE. These findings are supported by the results of a randomized controlled trial that compared enoxaparin with 2 different dosages of factor XI antisense oligonucleotide that specifically reduced factor XI levels, in patients undergoing elective unilateral total knee arthroplasty. The lower dosage was noninferior, and the higher dosage was superior to enoxaparin in reducing postoperative VTE. Factor XI antisense oligonucleotide appeared to be safe with respect to bleeding.23
It should be emphasized that patients with factor XI deficiency had a significantly higher prior history of gastrointestinal bleeding. However, no significant difference in the proportion of patients with prior history of intracerebral hemorrhage was observed between the 2 groups. The overall high proportion of prior bleeding detected in the study participants most likely reflects the tendency to test factor XI activity in patients with bleeding history.
This study has several advantages. First, it includes patients with a wide range of factor XI activity for whom outcomes and confounders data were available at the individual level, thus allowing us to examine dose-response relationship, adjust for confounder, and perform a more valid internal comparison with individuals with normal factor XI activity. Previous similar observational studies included only patients with severe factor XI deficiency and lacked adjustment for confounders, and outcome rates were compared with the national mean using nonconcurrent grouped data from a national survey.12-14 Second, our study is a population-based study including all individuals tested for factor XI activity in a large HMO (∼4.2 million members). Whereas previous studies included a small number of patients, ranging from 96 to 219 patients, who came from the same referral center for thrombosis and hemostasis disorders, they are raising concern about highly selective patients as a result of referral bias.12-14
The majority (77.7%) of the patients included in our study were young women in their childbearing age (median age 33.5 years; IQR, 27.8-42.7). At this age group, women are prone to bleeding episodes because of, for example, heavy menstrual bleeding and intrapartum hemorrhage, which could have led to excessive testing for factor XI activity. In addition, women in the childbearing age may undergo more routine blood tests, as part of routine follow-up during pregnancies, which might reveal elongated APTT and lead to further tests for factor XI activity. Restricting the analysis to women only showed similar results.
The major limitation of this historical cohort study is that included patients who were tested for factor XI activity during routine medical assessment most likely got tested because of an incidental laboratory finding or excessive bleeding. However, because of the rarity of factor XI deficiency, it would be impractical to perform a cohort study of randomly selected patients from the population. In addition, the higher prevalence of baseline comorbidities in patients with factor XI deficiency may raise concerns about selection bias in our study. However, this may be explained by the older age of factor XI–deficient patients in our study, which in turn is associated with a higher prevalence of comorbidities. Furthermore, selection bias is unlikely in this study because factor XI deficiency is genetically determined and cannot be predicted when tested for the same indication. The older age of factor XI–deficient patients suggests an interaction between age and factor XI on their effect on coagulation and fibrinolysis. Indeed factor XI deficiency remains masked for the greater portion of one’s life without evident clinical symptoms and only occasionally can present earlier on in life.10 Although the beneficial effect associated with factor XI deficiency seems to be mediated by the reduced procoagulant and antifibrinolytic activities, our study lacks other mechanistic coagulation analysis to provide a mechanism of cardiovascular and VTE protection associated with factor XI deficiency.
The findings of our study may have major therapeutic implications in subjects with an intact coagulation cascade; artificial means in the form of medication can be used to inflict factor XI deficiency in order to prevent cardiovascular morbidity and VTE. Our study suggests that this potential benefit may be achieved by inducing only mild–moderate factor XI deficiency without increasing the risk of bleeding associated with severe factor XI deficiency.
Conclusions
Factor XI deficiency is associated with a decreased incidence of cardiovascular events and VTE. Our study suggests a dose-response relationship between factor XI deficiency and VTE, and a J-shaped relationship between factor XI deficiency and cardiovascular events, with the greatest cardiovascular benefit achieved with mild–moderate factor XI deficiency. This study further supports the notion that inhibition, or lower activity levels of Factor XI, provides a cardiovascular benefit and a reduced VTE risk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.P. designed the research, analyzed the data, and wrote the manuscript; J.H. wrote the manuscript; A.K., A.Z., and N.S. designed the research and analyzed the data; G.R. analyzed the data and provided critical review of the manuscript; and W.S. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Meir Preis, Institute of Hematology, Lady Davis Carmel Medical Center, 7 Michal St, Haifa, Israel; e-mail: meirpr@clalit.org.il.