Key Points
Global survival rate was 74% at a median follow-up after HSCT of 57 months.
Preexisting mycobacterial infection and colitis were associated with poor HSCT outcome.
Abstract
X-linked recessive ectodermal dysplasia with immunodeficiency is a rare primary immunodeficiency caused by hypomorphic mutations of the IKBKG gene encoding the nuclear factor κB essential modulator (NEMO) protein. This condition displays enormous allelic, immunological, and clinical heterogeneity, and therapeutic decisions are difficult because NEMO operates in both hematopoietic and nonhematopoietic cells. Hematopoietic stem cell transplantation (HSCT) is potentially life-saving, but the small number of case reports available suggests it has been reserved for only the most severe cases. Here, we report the health status before HSCT, transplantation outcome, and clinical follow-up for a series of 29 patients from unrelated kindreds from 11 countries. Between them, these patients carry 23 different hypomorphic IKBKG mutations. HSCT was performed from HLA-identical related donors (n = 7), HLA-matched unrelated donors (n = 12), HLA-mismatched unrelated donors (n = 8), and HLA-haploidentical related donors (n = 2). Engraftment was documented in 24 patients, and graft-versus-host disease in 13 patients. Up to 7 patients died 0.2 to 12 months after HSCT. The global survival rate after HSCT among NEMO-deficient children was 74% at a median follow-up after HSCT of 57 months (range, 4-108 months). Preexisting mycobacterial infection and colitis were associated with poor HSCT outcome. The underlying mutation does not appear to have any influence, as patients with the same mutation had different outcomes. Transplantation did not appear to cure colitis, possibly as a result of cell-intrinsic disorders of the epithelial barrier. Overall, HSCT can cure most clinical features of patients with a variety of IKBKG mutations.
Introduction
X-linked recessive ectodermal dysplasia with immunodeficiency (XR-EDA-ID) is a rare primary immunodeficiency (PID) caused by hypomorphic mutations of the IKBKG gene, encoding nuclear factor κB (NF-κB) essential modulator (NEMO) a key regulator of the canonical NF-κB signaling pathway (Figure 1).1-3 NF-κB and NEMO are widely expressed and involved in many signal transduction pathways, including those downstream from interleukin 1 receptors, Toll-like receptors (TLRs), tumor necrosis factor receptors, vascular endothelial growth factor receptor-3, ectodysplasin-A receptor, and receptor activator of NF-κB (Figure 1).4 Loss-of-function mutations of IKBKG underlie X-linked incontinentia pigmenti (IP), which is lethal in male fetuses.4 In contrast, hypomorphic IKBKG mutations underlie XR-EDA-ID, which is characterized by diverse clinical manifestations, including EDA, life-threatening infections, and inflammatory diseases.1,2,5-7 A few patients also have osteopetrosis and lymphedema (XR-OL-EDA-ID).1,2,5-7 At least 3 nonhematopoietic pathways are affected: EDA results from alterations to the ectodysplasin/ectodysplasin-A receptor signaling pathway,5 lymphedema from changes to the vascular endothelial growth factor-3 pathway,1,2,5-7 and osteopetrosis from alterations to the receptor activator of NF-κB pathway.5 Conversely, some NEMO-deficient patients display no signs of EDA.1,2,5-10
NF-kB pathway. Immune receptor signaling pathways leading to NF-κB activation can be grouped into 4 categories on the basis of the surface receptors involved: developmental receptors (receptor activator of NF-κB, vascular endothelial growth factor receptor-3, and ectodysplasin-A receptor), antigen receptors (T-cell receptor [TCR] and B-cell receptor [BCR]), members of the TNF receptor superfamily (TNF-Rs: tumor necrosis factor receptors, CD40, FAS, etc), and members of the Toll-interleukin receptors superfamily (TIR: IL-1 receptors and TLRs). The protein of the NF-kB signaling pathway (NEMO) responsible for EDA-ID is shown in black.
NF-kB pathway. Immune receptor signaling pathways leading to NF-κB activation can be grouped into 4 categories on the basis of the surface receptors involved: developmental receptors (receptor activator of NF-κB, vascular endothelial growth factor receptor-3, and ectodysplasin-A receptor), antigen receptors (T-cell receptor [TCR] and B-cell receptor [BCR]), members of the TNF receptor superfamily (TNF-Rs: tumor necrosis factor receptors, CD40, FAS, etc), and members of the Toll-interleukin receptors superfamily (TIR: IL-1 receptors and TLRs). The protein of the NF-kB signaling pathway (NEMO) responsible for EDA-ID is shown in black.
The pathogenesis of the inflammatory and infectious phenotypes remains more elusive. B-cell and antibody (Ab) deficiencies include hypogammaglobulinemia, hyperimmunoglobulin (hyper-Ig) M and Ab deficiency.6,11,12 Most patients have normal T-cell counts and T-cell proliferation.6,9-12 Impaired NK cell cytotoxicity has been reported.6,11,12 The only immunologic abnormality consistently observed in these patients is a lack of glycan-specific Ab production, likely accounting for the high incidence of pneumococcal disease.1,2,5-7 XR-EDA-ID is perhaps the most heterogeneous PID yet identified at the allelic, immunological, and clinical levels,13 making clinical care of the patients particularly challenging. The precise mechanisms underlying the infectious and inflammatory phenotypes remain unknown, but hematopoietic stem cell transplantation (HSCT) has been performed in 29 children with IKBKG mutations9,10,12,14-30 (personal communications [see supplemental Methods, available on the Blood Web site]). These children probably account for less than 10% of the children with XR-EDA-ID reported worldwide.5 In contrast, 11 of the 14 reported patients with autosomal dominant EDA-ID, caused by gain-of-function mutations of NFKBIA, encoding IκBα, have undergone HSCT.16,31-41 This higher proportion of patients undergoing transplantation reflects both the greater severity and the homogeneity of this condition. We collectively considered 29 NEMO-deficient patients who underwent HSCT.1,9,10,12,14-28,30 This includes 10 previously unreported cases and has allowed for an updated analysis of the outcome of this procedure.
Methods
Patients and data collection
Patients with IKBKG hypomorphic mutations who underwent HSCT were studied.1,9,10,12,14-28,30,31,33,42-44 We collected published and unpublished cases from other physicians around the world (Table 1; supplemental Data).
Genetic and clinical features of the 29 NEMO-deficient patients before HSCT
| Patient . | Age at first symptom (months) . | IKBKG mutation . | Origin (country) . | EDA . | Rash . | Colitis . | O . | L . | Other symptoms . | References . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | c.1167_1168insC | USA | Yes | Yes | Yes | No | No | 16, 18; A. J. Mancini (pc) | |
| 2 | 2 | c.1237insT | France | Yes | No | Yes | Yes | No | C. Picard (pc) | |
| 3 | 0 | p.Q157P | USA | Yes | No | No | Yes | Yes | Anemia | 25; A. Jain (pc) |
| 4 | 6 | p.F312L | UK | Yes | Yes | No | No | No | W. Qasim (pc) | |
| 5 | 0 | p.L153R | USA | Yes | No | Yes | No | No | 12, 20, 41 | |
| 6 | 2 | c.1167-1168insC | Switzerland | Yes | Yes | No | Yes | No | HLH, developmental delay,* MGUS | 21; T. Gungor (pc) |
| 7 | 0 | p.G205X | Germany | Yes | Yes | No | No | No | 29; S. Ehl (pc) | |
| 8 | 2 | p.X420W | UK | Yes | No | Yes | Yes | Yes | 1, 14 | |
| 9 | 0 | c.768+5G>A | USA | Yes | No | Yes | No | Yes | 16, 19; J. Orange (pc) | |
| 10 | 30 | p.D311E | Japan | Yes | No | No | No | No | 22 | |
| 11 | 1.5 | c.1167_1168insC | USA | Yes | Yes | No | No | Yes | Delayed umbilical cord separation | 24; A. Filipovitch (pc) |
| 12 | 12 | p.R254Q | USA | No | No | No | No | No | AIHA | 24; A. Filipovitch (pc) |
| 13 | 9 | c.1245insT | UK | Yes | Yes | No | No | No | S. Jolles and M. Abinun (pc) | |
| 14 | 1.5 | c.1182_1183delTT | UK | Yes | No | NR | No | No | Erosive arthritis | W. Qasim (pc) |
| 15 | 9 | p.V146G | USA | Yes | Yes | Yes | No | No | 9, 27; E. W. Gelfand (pc) | |
| 16 | 6 | p.D113N | USA | No | No | No | No | No | 9, 10; E. W. Gelfand (pc) | |
| 17 | 12 | p.G348X | Japan | Yes | No | Yes | No | No | K. Imai and T. Okano (pc) | |
| 18 | 2 | c.1167-1168insC | USA | NR | NR | NR | No | No | J. Orange (pc) | |
| 19 | 1 | p.H413P | Brazil | yes | yes | yes | no | no | Ichthyosis vulgaris | 30; A. Condino (pc) |
| 20 | NR | p.Q391X | Japan | NR | NR | NR | NR | NR | K. Imai (pc) | |
| 21 | 3 | p.M407V | USA | No | No | NR | No | No | AIHA, neurological problem | 24; A. Filipovitch (pc) |
| 22 | NR | p.E315A | USA | Yes | No | No | Yes | No | Seizures (M avium brain lesion), lupus anticoagulant | 24; A. Filipovitch (pc) |
| 23 | 3 | p.A162P | New Zealand | Yes | No | Yes | No | No | Hashimoto thyroiditis | N. Cole (pc) |
| 24 | 4 | c.1167-1168insC | UK | Yes | Yes | Yes | No | No | A. Gennery (pc) | |
| 25 | 1.5 | c.1167_1168insC | Greece/Italy | Yes | Yes | Yes | Yes | Yes | 42 | |
| 26 | 0 | Duplication intron 3 to exon 6 | Japan | Yes | No | Yes | No | No | 23 | |
| 27 | 0 | c.1167_1168insC | Japan | Yes | Yes | Yes | No | No | Colic polyps | 15, 17; R. Nishikomori, T. Kawai (pc) |
| 28 | 1 | p.D306N | Mexico | Yes | Yes | Yes | No | No | ITP, intestinal vasculitis† | 28; L. Blancas (pc) |
| 29 | 1 | p.Q304-A305insDLP | France | Yes | Yes | Yes | No | No | S. Blanche (pc) |
| Patient . | Age at first symptom (months) . | IKBKG mutation . | Origin (country) . | EDA . | Rash . | Colitis . | O . | L . | Other symptoms . | References . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | c.1167_1168insC | USA | Yes | Yes | Yes | No | No | 16, 18; A. J. Mancini (pc) | |
| 2 | 2 | c.1237insT | France | Yes | No | Yes | Yes | No | C. Picard (pc) | |
| 3 | 0 | p.Q157P | USA | Yes | No | No | Yes | Yes | Anemia | 25; A. Jain (pc) |
| 4 | 6 | p.F312L | UK | Yes | Yes | No | No | No | W. Qasim (pc) | |
| 5 | 0 | p.L153R | USA | Yes | No | Yes | No | No | 12, 20, 41 | |
| 6 | 2 | c.1167-1168insC | Switzerland | Yes | Yes | No | Yes | No | HLH, developmental delay,* MGUS | 21; T. Gungor (pc) |
| 7 | 0 | p.G205X | Germany | Yes | Yes | No | No | No | 29; S. Ehl (pc) | |
| 8 | 2 | p.X420W | UK | Yes | No | Yes | Yes | Yes | 1, 14 | |
| 9 | 0 | c.768+5G>A | USA | Yes | No | Yes | No | Yes | 16, 19; J. Orange (pc) | |
| 10 | 30 | p.D311E | Japan | Yes | No | No | No | No | 22 | |
| 11 | 1.5 | c.1167_1168insC | USA | Yes | Yes | No | No | Yes | Delayed umbilical cord separation | 24; A. Filipovitch (pc) |
| 12 | 12 | p.R254Q | USA | No | No | No | No | No | AIHA | 24; A. Filipovitch (pc) |
| 13 | 9 | c.1245insT | UK | Yes | Yes | No | No | No | S. Jolles and M. Abinun (pc) | |
| 14 | 1.5 | c.1182_1183delTT | UK | Yes | No | NR | No | No | Erosive arthritis | W. Qasim (pc) |
| 15 | 9 | p.V146G | USA | Yes | Yes | Yes | No | No | 9, 27; E. W. Gelfand (pc) | |
| 16 | 6 | p.D113N | USA | No | No | No | No | No | 9, 10; E. W. Gelfand (pc) | |
| 17 | 12 | p.G348X | Japan | Yes | No | Yes | No | No | K. Imai and T. Okano (pc) | |
| 18 | 2 | c.1167-1168insC | USA | NR | NR | NR | No | No | J. Orange (pc) | |
| 19 | 1 | p.H413P | Brazil | yes | yes | yes | no | no | Ichthyosis vulgaris | 30; A. Condino (pc) |
| 20 | NR | p.Q391X | Japan | NR | NR | NR | NR | NR | K. Imai (pc) | |
| 21 | 3 | p.M407V | USA | No | No | NR | No | No | AIHA, neurological problem | 24; A. Filipovitch (pc) |
| 22 | NR | p.E315A | USA | Yes | No | No | Yes | No | Seizures (M avium brain lesion), lupus anticoagulant | 24; A. Filipovitch (pc) |
| 23 | 3 | p.A162P | New Zealand | Yes | No | Yes | No | No | Hashimoto thyroiditis | N. Cole (pc) |
| 24 | 4 | c.1167-1168insC | UK | Yes | Yes | Yes | No | No | A. Gennery (pc) | |
| 25 | 1.5 | c.1167_1168insC | Greece/Italy | Yes | Yes | Yes | Yes | Yes | 42 | |
| 26 | 0 | Duplication intron 3 to exon 6 | Japan | Yes | No | Yes | No | No | 23 | |
| 27 | 0 | c.1167_1168insC | Japan | Yes | Yes | Yes | No | No | Colic polyps | 15, 17; R. Nishikomori, T. Kawai (pc) |
| 28 | 1 | p.D306N | Mexico | Yes | Yes | Yes | No | No | ITP, intestinal vasculitis† | 28; L. Blancas (pc) |
| 29 | 1 | p.Q304-A305insDLP | France | Yes | Yes | Yes | No | No | S. Blanche (pc) |
AIHA, autoimmune hemolytic anemia; EDA, ectodermal anhidrotic dysplasia; HLH, hemophagocytic lymphohistiocytosis; ITP, immune thrombocytopenic purpura; L, lymphedema; MGUS, monoclonal gammopathy of undetermined significance; NR, not reported; O, osteopetrosis; pc, personal communication (see supplemental Data).
Subependymal calcifications on brain MRI.
Diagnosed on gut angiography.
Statistical analysis
Descriptive statistics are presented in the supplemental Data.
Results
Hemizygous IKBKG mutation and NEMO protein expression
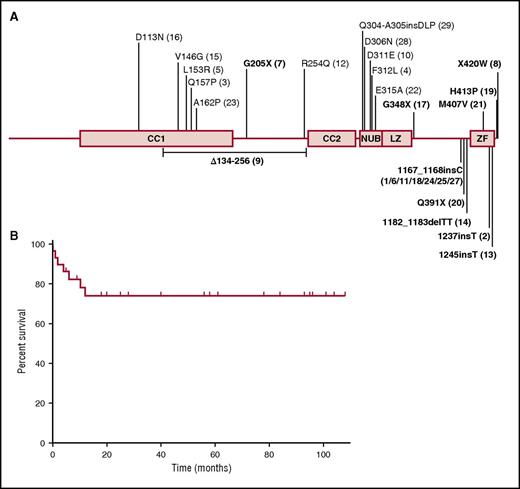
This series includes 19 patients for whom HSCT was reported in previous studies1,9,10,12,14-28,30,31,33,42-44 and 10 unreported patients. These 29 unrelated patients originated from 11 countries and were living in 9 countries (France, Germany, Switzerland, United Kingdom, Mexico, Brazil, United States, New Zealand, and Japan) (Table 1). These patients were hemizygous for 23 different IKBKG mutations (Figure 2A). All mutations were private to this cohort, with the exception of the c.1167_1168insC mutation, which was found in 7 unrelated patients. Functional testing was carried out for a total of 14 mutations, all of which were shown to be hypomorphic (supplemental Data). The mutations were missense (n = 13), nonsense (n = 3), small insertion (n = 4), small deletion (n = 1), large duplication (n = 1), and splice (n = 1) mutations (Table 1). Half the mutations affected the zinc finger (ZF) domain (n = 17). Six mutations affected coiled coil domain 1 or 2, and 5 mutations affected the NEMO ubiquitin-binding domain (Figure 2A). Flow cytometry or western blotting showed NEMO protein levels to be abnormally low in 4 of the 6 patients tested (P8, P10, P26, P27). NEMO protein levels were normal in P19 and P28, but specific assays highlighted changes to NEMO function.28,30 Functional assays were performed to test the NF-κB pathway specifically, in 17 patients (supplemental Data). Functional assays were not performed for 12 patients, 3 of whom carried previously described and tested mutations.
IKBKG mutations of the patients of this cohort and outcome after HSCT. (A) Schematic representation of the NEMO protein, with the various locations of the mutations found in the patients indicated. Patients with mutations affecting the zinc finger domain are shown in bold. The genetic defect of patient 26 (duplication of IKBKG intron 3 to exon 6) is not shown. (B) Overall survival rate of the 29 NEMO-deficient patients after HSCT. CC1, coiled coil domain 1; CC2, coiled coil domain 2; LZ, leucine zipper domain; NUB, NEMO ubiquitin-binding domain.
IKBKG mutations of the patients of this cohort and outcome after HSCT. (A) Schematic representation of the NEMO protein, with the various locations of the mutations found in the patients indicated. Patients with mutations affecting the zinc finger domain are shown in bold. The genetic defect of patient 26 (duplication of IKBKG intron 3 to exon 6) is not shown. (B) Overall survival rate of the 29 NEMO-deficient patients after HSCT. CC1, coiled coil domain 1; CC2, coiled coil domain 2; LZ, leucine zipper domain; NUB, NEMO ubiquitin-binding domain.
Carrier mothers and the developmental phenotype of the patients
Genetic analysis of the IKBKG coding region in the patients’ mothers found the same IKBKG mutation as in their affected sons, implying that none of the mutations occurred de novo, except in P24, who displayed mosaicism and whose mother did not carry the IKBKG mutation. When assessed, more than half these mothers presented mild IP symptoms. Almost all the women with IP symptoms carried mutations affecting the NEMO ZF domain. However, not all women carrying ZF mutations presented IP symptoms, suggesting phenotypic heterogeneity among women carrying the same mutation, possibly because of sporadic X inactivation.45-47 Mothers of P5 and P8 had IP. Five had inflammatory diseases: arthralgia (P1’s mother), colitis (P2’s mother), and recurrent mouth ulcers (the mothers of P3, P7, and P11). P6’s mother had recurrent bacterial infections. Twenty-four patients presented signs of EDA (Table 1). Only 3 patients (P12, P16, P21) had no signs of EDA. These patients were 7, 2, and 13 years old, respectively, at the time of HSCT. Consistent with these findings, the mutations of P12 and P16 had previously been described as associated with an absence of EDA in other patients.6 P21, whose mutation affects the ZF domain, does not have EDA either.24 Data were not available for P18 and P20. The 7 patients carrying the c.1167_1168insC mutation all had EDA. Six children presented with osteopetrosis (P2, P3, P6, P8, P22, P25): 5 patients with lymphedema (P3, P8, P9, P11, P25) and 3 patients with both features (Table 1).
Infectious phenotype of the patients
Median age at symptom onset was 2 months (range, birth-30 months) (Table 1). All patients except P2 and P29 had recurrent infections before HSCT (Table 2). P2 was diagnosed at birth because 1 of his older brothers had previously died of an invasive Pseudomonas aeruginosa infection in the context of EDA-ID with an IKBKG mutation. He thus received antibiotic prophylaxis and IV IgG (IVIG) from birth. P29 was diagnosed with hypogammaglobulinemia and low naive T-cell count, and thus received IVIG and antibiotic prophylaxis from age 1 month. No data were available for P16 and P20. The 25 remaining patients displayed severe recurrent bacterial infections, mostly as a result of pyogenic bacteria (Staphylococcus aureus [n = 9; 33%] and Streptococcus pneumoniae [n = 10; 37%]), Haemophilus influenzae b (n = 4; 14.8%), and other Gram-negative bacteria (n = 12; 44.4%). Sixteen patients had mycobacterial infection before HSCT: Mycobacterium avium (9/16), M fortuitum (2/16), M kansasii (1/16), M szulgai (1/16), and Bacille Calmette-Guérin vaccine (3/16). The mycobacterial species responsible for infection was not specified for P20. Severe viral infections were common (16/28) and were mostly caused by Herpesviridae sp (CMV [n = 6], EBV [n = 5], VZV [n = 2], HSV [n = 2], and HHV6 [n = 2]). Six patients developed hypoxic Pneumocystis pneumonia before the implementation of prophylaxis. Finally, 8 patients had other fungal infections, most commonly mucocutaneous candidiasis (n = 6).
Clinical and immunological phenotype of the 29 NEMO-deficient patients before HSCT
| Patient . | Pyogenic bacterial infection . | Fungal infection . | Mycobacterial infection . | Viral infection . | B cells . | T cells LPT to PHA . | NK cells cytotoxic activity . | ||
|---|---|---|---|---|---|---|---|---|---|
| IgG/A/M levels . | Posttetanic vaccine serology . | CD19+ CD27+ B-cell count . | |||||||
| 1 | Recurrent | No | M fortuitum | No | Low | Negative | NR | Normal | NR |
| 2 | No | No | No | No | Low | NR | NR | Normal | NR |
| 3 | Recurrent | P jirovecii | No | CMV | Low | NR | NR | NR | NR |
| 4 | Recurrent | No | M avium | HSV | Normal | NR | NR | Low | NR |
| 5 | Recurrent | No | No | CMV, MCV | Low IgG/M, hyper IgA | Normal | NR | Normal | Low |
| 6 | Recurrent | P jirovecii, Candida sp | M avium | CMV | Hyper IgM | NR | NR | Normal | Low |
| 7 | Recurrent | No | No | No | Low | NR | NR | Normal | Low |
| 8 | Recurrent | P jirovecii, C albicans | M kansasii | ADV | Low IgG | Normal | NR | Normal | Low |
| 9 | Recurrent | No | M avium | VZV (vaccine strain) | Low IgG, hyper IgM | Negative | Low | Normal | NR |
| 10 | Recurrent | No | M bovis | EBV | Low IgG | NR | NR | Normal | Normal |
| 11 | Recurrent | Metarhizium anisopliae | No | No | Low IgG, hyper IgM | NR | Low | Low | Low |
| 12 | Recurrent | No | M fortuitum | No | Low | Negative | Low | Normal | Low |
| 13 | Recurrent | No | M avium | ADV | Hyper IgA | NR | Low | Normal | Low |
| 14 | Recurrent | No | Yes (not specified) | No | Low | NR | NR | NR | NR |
| 15 | Recurrent | C parapsilosis | No | CMV | Low IgG, hyper IgA | Normal | NR | Normal | NR |
| 16 | NR | P jirovecii | No | CMV, rotavirus | Low IgA | Normal | NR | Normal | Low |
| 17 | Recurrent | Aspergillus niger | M avium, M bovis | No | NR | Negative | NR | Normal | NR |
| 18 | Recurrent | No | M avium | HHV6 | NR | NR | NR | NR | NR |
| 19 | Recurrent | C parapsilosis | M bovis | no | Low IgG, hyper IgM | Negative | NR | NR | NR |
| 20 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 21 | Recurrent | No | No | EBV | Low | Normal | Low | Normal | Low |
| 22 | Recurrent | No | M avium | No | NR | Negative | Low | NR | NR |
| 23 | Recurrent | P jirovecii | No | EBV, VZV | NR | NR | NR | NR | NR |
| 24 | Recurrent | P jirovecii | M avium | Sapovirus, EBV, HHV6 | Low IgG, hyper IgM | NR | NR | Normal | NR |
| 25 | Recurrent | No | No | No | Low IgG, hyper IgM | NR | NR | Normal | Low |
| 26 | One episode of sepsis | No | M szulgai | No | Low | NR | NR | Low | NR |
| 27 | Recurrent | C glabrata | No | No | Low | NR | NR | Normal | Low |
| 28 | Recurrent | C albicans | M avium | EBV, HSV | Normal | NR | NR | Normal | NR |
| 29 | Cutaneous abscess | No | No | CMV | Low IgG | Negative | Low | Normal | NR |
| Patient . | Pyogenic bacterial infection . | Fungal infection . | Mycobacterial infection . | Viral infection . | B cells . | T cells LPT to PHA . | NK cells cytotoxic activity . | ||
|---|---|---|---|---|---|---|---|---|---|
| IgG/A/M levels . | Posttetanic vaccine serology . | CD19+ CD27+ B-cell count . | |||||||
| 1 | Recurrent | No | M fortuitum | No | Low | Negative | NR | Normal | NR |
| 2 | No | No | No | No | Low | NR | NR | Normal | NR |
| 3 | Recurrent | P jirovecii | No | CMV | Low | NR | NR | NR | NR |
| 4 | Recurrent | No | M avium | HSV | Normal | NR | NR | Low | NR |
| 5 | Recurrent | No | No | CMV, MCV | Low IgG/M, hyper IgA | Normal | NR | Normal | Low |
| 6 | Recurrent | P jirovecii, Candida sp | M avium | CMV | Hyper IgM | NR | NR | Normal | Low |
| 7 | Recurrent | No | No | No | Low | NR | NR | Normal | Low |
| 8 | Recurrent | P jirovecii, C albicans | M kansasii | ADV | Low IgG | Normal | NR | Normal | Low |
| 9 | Recurrent | No | M avium | VZV (vaccine strain) | Low IgG, hyper IgM | Negative | Low | Normal | NR |
| 10 | Recurrent | No | M bovis | EBV | Low IgG | NR | NR | Normal | Normal |
| 11 | Recurrent | Metarhizium anisopliae | No | No | Low IgG, hyper IgM | NR | Low | Low | Low |
| 12 | Recurrent | No | M fortuitum | No | Low | Negative | Low | Normal | Low |
| 13 | Recurrent | No | M avium | ADV | Hyper IgA | NR | Low | Normal | Low |
| 14 | Recurrent | No | Yes (not specified) | No | Low | NR | NR | NR | NR |
| 15 | Recurrent | C parapsilosis | No | CMV | Low IgG, hyper IgA | Normal | NR | Normal | NR |
| 16 | NR | P jirovecii | No | CMV, rotavirus | Low IgA | Normal | NR | Normal | Low |
| 17 | Recurrent | Aspergillus niger | M avium, M bovis | No | NR | Negative | NR | Normal | NR |
| 18 | Recurrent | No | M avium | HHV6 | NR | NR | NR | NR | NR |
| 19 | Recurrent | C parapsilosis | M bovis | no | Low IgG, hyper IgM | Negative | NR | NR | NR |
| 20 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 21 | Recurrent | No | No | EBV | Low | Normal | Low | Normal | Low |
| 22 | Recurrent | No | M avium | No | NR | Negative | Low | NR | NR |
| 23 | Recurrent | P jirovecii | No | EBV, VZV | NR | NR | NR | NR | NR |
| 24 | Recurrent | P jirovecii | M avium | Sapovirus, EBV, HHV6 | Low IgG, hyper IgM | NR | NR | Normal | NR |
| 25 | Recurrent | No | No | No | Low IgG, hyper IgM | NR | NR | Normal | Low |
| 26 | One episode of sepsis | No | M szulgai | No | Low | NR | NR | Low | NR |
| 27 | Recurrent | C glabrata | No | No | Low | NR | NR | Normal | Low |
| 28 | Recurrent | C albicans | M avium | EBV, HSV | Normal | NR | NR | Normal | NR |
| 29 | Cutaneous abscess | No | No | CMV | Low IgG | Negative | Low | Normal | NR |
ADV, adenovirus; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV6, human herpesvirus 6; HSV, herpes simplex virus; LPT, lymphocyte proliferation test; MCV, Molluscum contagiosum virus; NR, not reported; P jirovecii, Pneumocystis jirovecii; VZV, varicella zoster virus.
Inflammatory and autoimmune diseases
Thirteen patients had inflammatory skin involvement before HSCT, with early skin rash resulting from eczematous dermatitis. Skin biopsy was performed in 8 patients, revealing spongiotic dermatitis with superficial perivascular inflammatory lymphocytes and eosinophil infiltrates. Skin involvement was treated with topical corticosteroids, but systemic corticosteroids or cyclosporin (P1, P25) treatment were administered in severe cases. Fifteen patients had symptoms of inflammatory bowel disease (IBD) before HSCT. Eight of these cases were confirmed by a gut biopsy showing nonspecific inflammation characterized by neutrophilic infiltrates without granuloma, crypt abscesses, glandular destruction, and crypt regeneration. Four children received immunosuppressive treatment before HSCT (P1, P5, P25, P29). P6 developed secondary hemophagocytic lymphohistiocytosis concomitantly with bacterial sepsis, which resolved with recovery from infection. The following autoimmune additional features were observed: cytopenia (hemolytic autoimmune anemia for P12 and P21, and immune thrombocytopenic purpura for P28), lupus anticoagulant (asymptomatic, P22), and Hashimoto’s thyroiditis (P23; Table 1).
Immunological phenotype
Immunological investigations before HSCT revealed heterogeneous abnormalities. Hypogammaglobulinemia was common among these patients (19/24). Six patients presented with hyper-IgM (P6, P9, P11, P19, P24, P25). These patients carried the Δ134-256 (P9), c.1167_1168insC (P6, P11, P24, P25), or H413P mutations (P19), leading to changes to NEMO protein domains already implicated, to various extents, in hyper-IgM phenotype.6 Posttetanus immunization-specific Abs were below protective levels in half of those assayed (7/12), and low memory CD27+/CD19+ B-cell counts persisted in all assessed patients (7/7). CD4 or CD8 T lymphocytosis was also observed in 7 patients. Normal results were obtained in tests of lymphocyte proliferation in response to mitogens in all but 3 of the children (P4, P11, P26; 3/22). The cytotoxic activity of NK cells was poor (11/12) (Table 2).
Management before HSCT
Most children needed nutritional support before HSCT (13/18), and 5 required parenteral nutrition. Twenty-four patients received IVIG, and 22 received trimethoprim-sulfamethoxazole prophylaxis against bacterial infections. Ten patients received azithromycin or clarithromycin for prophylaxis against mycobacterial infection. All patients with mycobacterial disease received combined antimycobacterial antibiotic therapy, and 4 of them received interferon (IFN)-γ (P8, P13, P18, P22). Ten children were treated with antiviral drugs. Two patients were receiving cyclosporin A and corticosteroids for suspected maternofetal graft-versus-host disease (GVHD) during the neonatal period (P25, P27). Four patients received immunosuppressive drugs to treat colitis (corticosteroids and infliximab for P1, azathioprine and sulfalazine for P5, and infliximab for P29), and P28 was treated with corticosteroids, cyclosporin A, rituximab, and thalidomide for refractory immune thrombocytopenic purpura.
HSCT donor and conditioning regimens
Most of the HSC donors were unrelated donors (UDs; 20/29; 68.9%). Twelve were HLA-matched unrelated donors (MUDs), and 8 were mismatched unrelated donors. Seven donors were matched sibling donors (MSDs), including 5 sisters, 3 of whom carried IKBKG mutation (P5-P7; Table 3). Two donors had symptoms (IP for P6’s donor; recurrent mouth ulcers, uveitis, and erythema nodosum for P7’s donor). P28 and P29’s donors were their mother, haploidentical carriers (T-depleted graft for P28 and T-replete graft for P29). The sources of HSCs used were bone marrow (n = 13), cord blood (n = 6), peripheral blood SC (n = 3), and not specified (n = 5 patients). The conditioning regimens (supplemental Data) for the first HSCT were mostly classical myeloablative regimens (15/28). A reduced intensity conditioning (RIC) regimen was used in 13 children (Table 3). Eight cases of severe conditioning toxicity were reported: tubulopathy (P6), severe venoocclusive disease (P7), severe hypotension during antithymocyte globulin infusion followed by fatal venoocclusive disease (P8), profuse diarrhea (P9), fatal intracranial bleeding (P18), P aeruginosa septicemia (P24), hepatic toxicity after methotrexate infusion (P27), and severe mucositis (P7, 13). Almost all patients with severe conditioning regimen adverse effects carried mutations affecting the ZF domain (7/8), resulting in a higher frequency of conditioning regimen toxicity in patients with such mutations than in patients with mutations affecting other domains (39% vs 9%; P = .1). Four children underwent a second HSCT because of graft failure (P1, P9, P12, P19). The second HSCT was performed with peripheral blood SC from the same donor as for the first HSCT in all cases, using RIC for P1, fludarabine alone for P9, and no conditioning for P12 (data not available for P19; Table 3).
HSCT of the 29 NEMO-deficient patients
| Patient . | Age at HSCT (y) . | HLA match . | Source . | MNC (×108 cells/kg) . | Conditioning regimen . | Day ANC>500 . | GVHD prophylaxis . | Donor chimerism (%) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.41 | MSD | PSC | 6.2 | Flu-Bu-rATG | 17 | NR | 19 |
| 1bis | 1.25 | MSD† | PSC | 6 | Flu-Mel-Alz | 16 | NR | 98 |
| 2 | 0.33 | MSD | BM | 4.03 | Flu-Bu-rATG | 19 | CsA-MMF | 85 |
| 3 | 0.41 | MSD | BM | NR | Flu-TBI | NR | NR | 86 |
| 4 | 5.4 | MSD | NR | NR | Bu-Flu-rATG | NR | Csa-CST | 100 |
| 5 | 5.41 | MSD* | BM/CB | 3.63 | Bu-Cy | 14 | CST-CsA | 98 |
| 6 | 6.33 | MSD* | BM/CB | NR | Flu-Bu-Alz | 29 | CsA | 99 |
| 7 | 0.75 | MSD* | BM | NR | Bu-Cy- rATG | NR | CST-CsA | 100 |
| 8 | 1.41 | MUD | BM | NR | Bu-Cy- rATG | None | NR | NR |
| 9 | 3.41 | MUD | BM | 0.25 | Bu-Cy- rATG | None | None | 65 |
| 9bis | 3.58 | MUD† | PSC | 0.1 | Flu | 12 | None | 100 |
| 10 | 4.9 | MUD | BM | 5.9 | Flu-Mel-rATG | 10 | Tacro-MMF-CST | 100 |
| 11 | 3.3 | MUD | BM | 6.12 | Bu-Cy-eATG | 9 | CST-CsA | 100 |
| 12 | 7.75 | MUD | BM | 7.2 | Bu-Cy- rATG | 11 | CST-CsA | 60 |
| 12bis | 12.5 | MUD† | PSC | NR | No | NR | No | 53 |
| 13 | 6 | MUD | BM | 6.6 | Flu-Mel-rATG | 14 | CsA-MMF | 100 |
| 14 | 8 | MUD | PSC | 19.2 | Flu-Mel-Alz | NR | NR | 82 |
| 15 | 2.4 | MUD | CB | NR | Bu-Cy- rATG | 15 | CsA-MMF | 100 |
| 16 | 2.1 | MUD | CB | NR | Bu-Cy-eATG | NR | CsA-MMF | 100 |
| 17 | 12.25 | MUD | NR | NR | Flu-Bu-rATG | NR | NR | 100 |
| 18 | NR | MUD | NR | NR | Bu-Cy | NR | NR | NR |
| 19 | 2.25 | MUD | NR | NR | NR | NR | Yes (unspecified) | 80 |
| 19bis | 2.5 | MUD† | PSC | NR | NR | NR | No | NR |
| 20 | 1 | MUD 9/10 | NR | NR | Bu-Flu-rATG | NR | Tacro-MTX | NR |
| 21 | 13.5 | MUD 7/8 | BM | 3.33 | Bu-Cy-eATG | 11 | CST-CsA | 100 |
| 22 | 18.83 | MUD 7/8 | BM | 3.94 | Bu-Cy-eATG | 14 | CST-CsA | 100 |
| 23 | 3.8 | MUD 5/6 | CB | 6.9 | Bu-Cy-eATG | NR | CsA-MMF | 100 |
| 24 | 4.5 | MUD 8/10 | BM | 5.9 | Flu-Bu-Alz | 15 | CsA-MMF | 100 |
| 25 | 0.62 | MUD 7/10 | CB | 1.19 | Bu-Cy-eATG | 20 | CST-CsA | 100 |
| 26 | 2.16 | MUD 5/8 | CB | 0.85 | Flu-Mel-rATG | 13 | Tacro-then CsA | 30 |
| 27 | 3 | MUD 2/6 | CB | 0.6 | Flu-Mel-rATG | 19 | MTX J1/3-tacro | 100 |
| 28 | 13.83 | Haplo id* | PSC | 4 | Flu-Mel-rATG | 10 | CST-CsA | NR |
| 29 | 0.9 | Haplo id* | BM | 13 | Flu-Bu-Alz | 37 | Cy-CsA-MMF | 100 |
| Patient . | Age at HSCT (y) . | HLA match . | Source . | MNC (×108 cells/kg) . | Conditioning regimen . | Day ANC>500 . | GVHD prophylaxis . | Donor chimerism (%) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.41 | MSD | PSC | 6.2 | Flu-Bu-rATG | 17 | NR | 19 |
| 1bis | 1.25 | MSD† | PSC | 6 | Flu-Mel-Alz | 16 | NR | 98 |
| 2 | 0.33 | MSD | BM | 4.03 | Flu-Bu-rATG | 19 | CsA-MMF | 85 |
| 3 | 0.41 | MSD | BM | NR | Flu-TBI | NR | NR | 86 |
| 4 | 5.4 | MSD | NR | NR | Bu-Flu-rATG | NR | Csa-CST | 100 |
| 5 | 5.41 | MSD* | BM/CB | 3.63 | Bu-Cy | 14 | CST-CsA | 98 |
| 6 | 6.33 | MSD* | BM/CB | NR | Flu-Bu-Alz | 29 | CsA | 99 |
| 7 | 0.75 | MSD* | BM | NR | Bu-Cy- rATG | NR | CST-CsA | 100 |
| 8 | 1.41 | MUD | BM | NR | Bu-Cy- rATG | None | NR | NR |
| 9 | 3.41 | MUD | BM | 0.25 | Bu-Cy- rATG | None | None | 65 |
| 9bis | 3.58 | MUD† | PSC | 0.1 | Flu | 12 | None | 100 |
| 10 | 4.9 | MUD | BM | 5.9 | Flu-Mel-rATG | 10 | Tacro-MMF-CST | 100 |
| 11 | 3.3 | MUD | BM | 6.12 | Bu-Cy-eATG | 9 | CST-CsA | 100 |
| 12 | 7.75 | MUD | BM | 7.2 | Bu-Cy- rATG | 11 | CST-CsA | 60 |
| 12bis | 12.5 | MUD† | PSC | NR | No | NR | No | 53 |
| 13 | 6 | MUD | BM | 6.6 | Flu-Mel-rATG | 14 | CsA-MMF | 100 |
| 14 | 8 | MUD | PSC | 19.2 | Flu-Mel-Alz | NR | NR | 82 |
| 15 | 2.4 | MUD | CB | NR | Bu-Cy- rATG | 15 | CsA-MMF | 100 |
| 16 | 2.1 | MUD | CB | NR | Bu-Cy-eATG | NR | CsA-MMF | 100 |
| 17 | 12.25 | MUD | NR | NR | Flu-Bu-rATG | NR | NR | 100 |
| 18 | NR | MUD | NR | NR | Bu-Cy | NR | NR | NR |
| 19 | 2.25 | MUD | NR | NR | NR | NR | Yes (unspecified) | 80 |
| 19bis | 2.5 | MUD† | PSC | NR | NR | NR | No | NR |
| 20 | 1 | MUD 9/10 | NR | NR | Bu-Flu-rATG | NR | Tacro-MTX | NR |
| 21 | 13.5 | MUD 7/8 | BM | 3.33 | Bu-Cy-eATG | 11 | CST-CsA | 100 |
| 22 | 18.83 | MUD 7/8 | BM | 3.94 | Bu-Cy-eATG | 14 | CST-CsA | 100 |
| 23 | 3.8 | MUD 5/6 | CB | 6.9 | Bu-Cy-eATG | NR | CsA-MMF | 100 |
| 24 | 4.5 | MUD 8/10 | BM | 5.9 | Flu-Bu-Alz | 15 | CsA-MMF | 100 |
| 25 | 0.62 | MUD 7/10 | CB | 1.19 | Bu-Cy-eATG | 20 | CST-CsA | 100 |
| 26 | 2.16 | MUD 5/8 | CB | 0.85 | Flu-Mel-rATG | 13 | Tacro-then CsA | 30 |
| 27 | 3 | MUD 2/6 | CB | 0.6 | Flu-Mel-rATG | 19 | MTX J1/3-tacro | 100 |
| 28 | 13.83 | Haplo id* | PSC | 4 | Flu-Mel-rATG | 10 | CST-CsA | NR |
| 29 | 0.9 | Haplo id* | BM | 13 | Flu-Bu-Alz | 37 | Cy-CsA-MMF | 100 |
The “bis” for patients 1, 9, 12 and 19 corresponds to the second HSCT. MUD mismatches are specified.
Alz, alemtuzumab; ANC >500, absolute neutrophil count >500/mm3; ATG, antithymocyte globulin (e, horse; r, rabbit); BM, bone marrow stem cell graft; Bu, busulfan; CB, cord blood stem cell graft; CsA, cyclosporin A; CST, corticosteroids; Cy, cyclophosphamide; Flu, fludarabine; haplo-id, haplo-identical donor; Mel, melphalan; MMF, mycophenolate mofetil; MNC, mononuclear cell count; MTX, methotrexate; PSC, peripheral blood stem cells; Tacro, tacrolimus; TBI, total body irradiation; y, years.
Carrier sister or mother.
Same donor as for the first HSCT.
HSCT engraftment
The median count of first graft mononuclear cells was 4.96 × 108/kg (range, 0.25-19.2 × 108/kg). Donor leukocyte chimerism after first HSCT mostly exceeded 90% (20/23). Chimerism could not be evaluated in 4 children who died less than 2 months after HSCT (P8, P18, P19, P26), and data were not available for 2 patients. The median time between the first graft and the attainment of an absolute neutrophil count above 500/mm3 was 14 days (range, 9-37 days), excluding the cases of early death (P8 died on day 6) and early graft failure (P9). Among the 4 patients who suffered graft rejection, 2 patients required an early boost infusion of CD34+ cells less than 3 months after the first HSCT (P9, P19); they both died after the second HSCT because of respiratory viral infection (P9, partial lymphoid engraftment) or septic shock (P19). The other 2 children, P1 and P12, received boosts 10 and 57 months after the first HSCT, respectively. The second HSCT was successful in both cases. The type of conditioning regimen did not influence graft failure occurrence (7.7% among RIC conditioned patients vs 13.3% among classical myeloablative regimens conditioned children group; P = .55; data not available for P19). All patients received post-HSCT GVHD prophylaxis except P9, who suffered graft failure, and P28, who received a T-depleted haplo-identical graft. The GVHD prophylaxis regimens consisted of corticosteroids and cyclosporin A (9/20) or mycophenolate mofetil and cyclosporin A (7/20). P29 received cyclophosphamide after his T-replete haploidentical graft. Acute GVHD occurred in half of the patients (13/27), but had no effect on survival rate (supplemental Figure 1A). Most cases of involved the skin (8/10), with progression to chronic skin GVHD in 2 patients, as well as the gut (3/9) and liver (1/9). Most cases were unipolar (9/11) and of low grade (grade I or II in 7 of 11 specified cases; Table 4).
HSCT outcome of the 29 NEMO-deficient patients
| Patient . | GVHD . | Symptoms after HSCT . | Status . | Cause of death . |
|---|---|---|---|---|
| 1 | Yes | Graft failure after first HSCT | Alive | |
| 1bis | No | Partial cutaneous improvement, colitis, brain pseudotumor | ||
| 2 | No | Warts | Alive | |
| 3 | Yes | Skin rash | Alive | |
| 4 | Yes | None | Alive | |
| 5 | Yes | Colitis, CMV reactivation | Alive | |
| 6 | No | VZV encephalitis and bacterial infections | Alive | |
| 7 | No | Severe colitis | Alive | |
| 8 | No | VOD | Dead | VOD |
| 9 | No | Graft failure after first HSCT | Dead | ARDS (parainfluenza virus infection) |
| 9bis | No | Bacterial and viral infections, colitis | ||
| 10 | Yes | Multiple papillomas virus | Alive | |
| 11 | Yes | Bacterial and viral infections | Alive | |
| 12 | Yes | Graft failure after first HSCT | Alive | |
| 12bis | No | CMV, EBV, and ADV reactivation | ||
| 13 | No | Warts, VZV encephalopathy | Alive | |
| 14 | No | Colitis, chronic lung disease, arthropathy, arteriopathy* | Alive | |
| 15 | No | None | Alive | |
| 16 | No | Allergies to sulfamides and walnuts | Alive | |
| 17 | NR | BK virus cystitis | Alive | |
| 18 | NR | Intracranial bleeding | Dead | Intracranial bleeding |
| 19 | No | Graft failure after first HSCT | Dead | Septic shock |
| 19bis | No | Bacterial septic shock | ||
| 20 | No | Transient idiopathic intracranial hypertension | Alive | |
| 21 | No | EBV viremia | Alive | |
| 22 | No | Mycobacterial disseminated infection, HSV pneumonitis | Dead | Disseminated mycobacterial infection |
| 23 | Yes | Bacterial infection | Alive | |
| 24 | Yes | Colitis, Pseudomonas infection | Alive | |
| 25 | No | Severe RSV infection 1 y post HSCT | Alive | |
| 26 | Yes | Bacterial septic shock | Dead | Septic shock |
| 27 | Yes | None | Alive | |
| 28 | Yes | Bacterial septic shock | Dead | Septic shock |
| 29 | Yes | CMV reactivation, colitis | Alive |
| Patient . | GVHD . | Symptoms after HSCT . | Status . | Cause of death . |
|---|---|---|---|---|
| 1 | Yes | Graft failure after first HSCT | Alive | |
| 1bis | No | Partial cutaneous improvement, colitis, brain pseudotumor | ||
| 2 | No | Warts | Alive | |
| 3 | Yes | Skin rash | Alive | |
| 4 | Yes | None | Alive | |
| 5 | Yes | Colitis, CMV reactivation | Alive | |
| 6 | No | VZV encephalitis and bacterial infections | Alive | |
| 7 | No | Severe colitis | Alive | |
| 8 | No | VOD | Dead | VOD |
| 9 | No | Graft failure after first HSCT | Dead | ARDS (parainfluenza virus infection) |
| 9bis | No | Bacterial and viral infections, colitis | ||
| 10 | Yes | Multiple papillomas virus | Alive | |
| 11 | Yes | Bacterial and viral infections | Alive | |
| 12 | Yes | Graft failure after first HSCT | Alive | |
| 12bis | No | CMV, EBV, and ADV reactivation | ||
| 13 | No | Warts, VZV encephalopathy | Alive | |
| 14 | No | Colitis, chronic lung disease, arthropathy, arteriopathy* | Alive | |
| 15 | No | None | Alive | |
| 16 | No | Allergies to sulfamides and walnuts | Alive | |
| 17 | NR | BK virus cystitis | Alive | |
| 18 | NR | Intracranial bleeding | Dead | Intracranial bleeding |
| 19 | No | Graft failure after first HSCT | Dead | Septic shock |
| 19bis | No | Bacterial septic shock | ||
| 20 | No | Transient idiopathic intracranial hypertension | Alive | |
| 21 | No | EBV viremia | Alive | |
| 22 | No | Mycobacterial disseminated infection, HSV pneumonitis | Dead | Disseminated mycobacterial infection |
| 23 | Yes | Bacterial infection | Alive | |
| 24 | Yes | Colitis, Pseudomonas infection | Alive | |
| 25 | No | Severe RSV infection 1 y post HSCT | Alive | |
| 26 | Yes | Bacterial septic shock | Dead | Septic shock |
| 27 | Yes | None | Alive | |
| 28 | Yes | Bacterial septic shock | Dead | Septic shock |
| 29 | Yes | CMV reactivation, colitis | Alive |
The “bis” for patients 1, 9, 12 and 19 corresponds to the second HSCT.
ARDS, acute respiratory distress syndrome; RSV, respiratory syncytial virus.
Unexplained cerebral arteriopathy of the circle of Willis requiring surgical bypass.
Outcome after HSCT
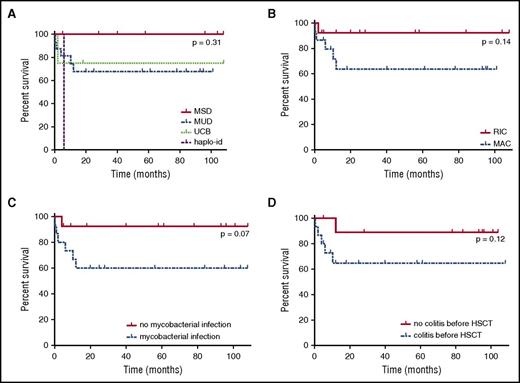
Median age at the time of first HSCT was 3.35 years (range, 4 months-18.8 years). The overall survival rate was 74% at 108 months after transplantation (Figure 2B), with a median follow-up of 57 months in surviving children (range, 4-108 months). Surprisingly, age at transplantation did not seem to influence the survival rate (supplemental Figure 1B). All patients receiving HSCs from a MSD were still alive at the end of follow-up, whereas only 68.5% of children undergoing HSCT with HSCs from an UD survived. Overall, there were no statistically significant differences regarding the type of HSC donor (survival rate among MSD graft: 100% at 108 months; MUD graft: 67.7% at 108 months; UCB graft: 75% at 108 months; haplo-identical graft: 50% at 5 months; P = 0.31; Figure 3A). There was also no difference in survival between children receiving grafts from MUDs and children receiving grafts from mismatched UDs (66.7% at 101 months and 70% at 108 months, respectively [P = .72]; data not shown). The choice of a classical myeloablative regimen or RIC regimen had no significant effect on survival rate after HSCT (63.5% at 101 months vs 92.3% at 108 months, respectively, P = .14; Figure 3B). Seven patients died after HSCT, a median of 2 months after the last HSCT (range, 0.2-12 months). Severe infection was a major cause of death in this population: 3 patients died of bacterial septic shock, 1 from a severe viral respiratory infection, and another from disseminated M avium complex infection (documented before HSCT). Two other children died of conditioning regimen toxicity (venoocclusive disease and intracranial bleeding; Table 4). The children who died received grafts with significantly lower mononuclear cell counts than those of the other children (2.39 × 108/kg vs 6.01 × 108/kg [P = .02]; data not shown). Mycobacterial infection before HSCT seemed to decrease survival rate, as only 60% of the children with mycobacterial infection were alive after HSCT vs 92.3% of those without (Figure 3C; P = .07). In contrast, viral and fungal infections before HSCT did not influence survival rate after HSCT (supplemental Figure 1D-E). Bacterial infections recurred after HSCT in 30% of the patients with successful engraftment (8/27), but only 2 of these patients had infections more than 6 months after HSCT (P6: pneumococcal meningitis and H influenzae infection; P12: sinusitis).
Survival rate of NEMO-deficient patients after HSCT. (A) Survival rate after HSCT, by donor type. MUD, blue dotted line; MSD, red full line; UCB, matched unrelated cord blood donor, green regular small dotted line; haplo-id: haploidentical donor, purple regular large dotted line. (B) Survival rate after HSCT by conditioning regimen. MAC, blue dotted line; RIC, red full line. (C) Survival rate after HSCT, by presence or absence of mycobacterial infection before HSCT. The red full line corresponds to patients with no mycobacterial infection, and the blue dotted line corresponds to patients with mycobacterial infection before HSCT. (D) Survival rate after HSCT, by presence or absence of colitis before HSCT. The red full line corresponds to patients without colitis, and the blue dotted line corresponds to patients with colitis before HSCT.
Survival rate of NEMO-deficient patients after HSCT. (A) Survival rate after HSCT, by donor type. MUD, blue dotted line; MSD, red full line; UCB, matched unrelated cord blood donor, green regular small dotted line; haplo-id: haploidentical donor, purple regular large dotted line. (B) Survival rate after HSCT by conditioning regimen. MAC, blue dotted line; RIC, red full line. (C) Survival rate after HSCT, by presence or absence of mycobacterial infection before HSCT. The red full line corresponds to patients with no mycobacterial infection, and the blue dotted line corresponds to patients with mycobacterial infection before HSCT. (D) Survival rate after HSCT, by presence or absence of colitis before HSCT. The red full line corresponds to patients without colitis, and the blue dotted line corresponds to patients with colitis before HSCT.
IBD after HSCT
Five of the 15 patients presenting with IBD before transplantation died after HSCT, 4 patients had persistent or intermittent colitis (P1, P5, P24, P29), and IBD symptoms disappeared in 6 patients. IBD occurred de novo post-HSCT in 2 previously unaffected patients without pathological evidence of gastrointestinal GVHD (P7, P14). Gut biopsies were performed in P7, highlighting cell tufting in the small intestine and multiple large ulcers in the large intestine. Moreover, gut-infiltrating immune cells were strongly stained for nuclear p65, indicating normal NEMO function, whereas intestinal epithelial cells had no nuclear p65, suggesting the possible involvement of an intrinsic NF-κB signaling defect in intestinal epithelial cells.29 No biopsy results were reported for P14. P7 and P14 were both treated with corticosteroids and infliximab (with high doses of infliximab for P7). Post-HSCT outcome seemed to be worse among children suffering from IBD before HSCT, only 64.6% of whom were still alive after HSCT vs 88.9% of the patients without gastrointestinal symptoms before the graft. However, this difference was not statistically significant (Figure 3D; P = .12).
Immunological reconstitution after HSCT
Immunological reconstitution was assessed in the 22 surviving patients. Data were unavailable for 7 patients (P1, P3, P4, P17, P20, P24, P27) or cannot be assessed (P29, last follow-up only few months after graft). NEMO protein levels in PBMCs were restored to normal (2/2), as was the production of cytokines by PBMCs in response to TLR ligands (3/3) and NK cell cytotoxicity (3/3). Complete B-cell reconstitution (B-cell subsets and Ig levels) occurred in almost all children (11/13). B-cell proliferation in response to CD40 stimulation recovered after HSCT (3/3). Only 2 patients were still on IVIG 95 and 37 months after HSCT (P12, P14), 1 of whom was treated with immunosuppressive drugs for a recurrence of severe inflammatory colitis (P14, corticosteroids and infliximab). Both have partial chimerism on B cells.
Discussion
We report HSCT outcome for an international cohort of 29 NEMO-deficient patients with various forms of EDA-ID, representing the largest cohort assembled to date. Because data were retrospectively collected, some patient characteristics and complications may be underestimated, and the follow-up of the children may be heterogeneous, leading to missing data. In this cohort, 59% of the patients had mutations affecting the ZF domain of NEMO. This domain is a highly conserved structural motif involved in the recognition of upstream regulators of the IKK complex; it is essential for NF-κB activation in various immune cell types and in ectoderm-derived cells.48,49 Mutations affecting the ZF domain have been associated with a more severe EDA-ID phenotype, including a higher frequency of EDA, osteopetrosis, IBD, pyogenic bacterial infections, and mycobacterial susceptibility.5,6 As HSCT is generally considered only for the sickest patients, this may account for the high proportion of patients with ZF domain mutations in this cohort. Nevertheless, a comparison of this cohort with the patients reported by Hanson et al revealed a similar proportion of patients with ZF domain mutations, but a significantly higher frequency of inflammatory symptoms, IBD, and chronic viral infections among our patients (supplemental Table 1). These observations suggest that the IKBKG genotype is important, but that other individual or environmental cofactors may modify individual inflammatory and infectious complications. The IKBKG genotype may be considered when discussing the case for HSCT in NEMO-deficient patients, but is not sufficient to be an indication for HSCT in itself.
A previous report suggested there were intrinsic difficulties with HSCT engraftment in patients with EDA-ID.50 We did not find this to be the case in this study, for which the global engraftment rate was 93%. Five patients received grafts from a carrier sister or mother. One of these patients had recurrent infections resulting from encapsulated bacteria after HSCT (P6), 2 had persistent intermittent colitis (P5, P29), and the third developed de novo colitis (P7). In contrast, no bacterial infections occurred in the 4 children receiving HSCs from MSDs without IKBKG mutations; only 1 of these patients had persistent colitis (P1). This suggests HSCs from a carrier sister or mother may only partially correct the susceptibility to bacterial infections, possibly as a result of random X-linked IKΒΚG inactivation in some types of leukocytes, leading to a deficiency in half the donor immune cells.45-47 Moreover, X inactivation may be skewed in the leukocytes of the IKBKG mutation carriers, potentially amplifying the deficiency. Consistent with this hypothesis, skewed X inactivation favored the mutant allele in the carrier sister acting as the donor for P7 (only 26% PBMCs expressed the wild-type allele), and this skewed X inactivation was more pronounced in the recipient, P7, in whom only 10% of PBMCs expressed the wild-type allele.29 We report only 4 cases, too small a number of patients for any firm conclusions to be drawn. However, it would be interesting to study X inactivation in the PBMCs of the carrier sisters acting as donors and in the recipients, to determine whether this process affects HSCT outcome (Bustamante et al in preparation).
Global survival was 74%, a rate similar to those reported for HSCT in other non-SCID PIDs (79% at 3 years).51 Remarkably, all deaths occurred early, during the first year after HSCT, and most were a result of severe infections, suggesting a role of induced immunosuppression post-HSCT and infectious status before grafting. Mycobacterial infection has been shown to be associated with poor prognosis in patients not undergoing transplantation.6,50 In this series, mycobacterial infection before HSCT seemed to be associated with greater post-HSCT mortality, although this difference was not statistically significant. This observation may reflect an inability to clear mycobacterial infections effectively before HSCT. Four of the 16 patients with mycobacterial infections before HSCT received IFN-γ therapy, including 3 patients who died after HSCT, 1 from disseminated mycobacterial disease. The number of patients treated was too small to determine whether IFN-γ therapy itself influenced the prognosis. Surprisingly, 1 patient died of fatal intracranial bleeding soon after transplantation, even though he did not have severe thrombocytopenia (platelet count remained above 20 000/mm3). It is possible that this bleeding results from vascular instability caused by aberrant NF-κB pathways in the vascular endothelium. Indeed, fundamental studies have suggested the NF-κB pathway is involved in promoting the proliferation of endothelial progenitor cells.52 Moreover, NF-κB signaling is essential for the TNF-α activation of endothelial cells and the resulting regulation of vascular permeability and control of inflammatory responses in endothelial cells.53 Consistent with this hypothesis, P20 developed an unexplained cerebral arteriopathy of the circle of Willis after HSCT, and P26 had intestinal vasculitis before HSCT, suggesting the involvement of endothelial cells. These findings suggest more attention should be paid to platelet levels during HSCT for NEMO-deficient patients. It seems to be important to keep a higher threshold for platelet transfusion in NEMO-deficient patients undergoing HSCT to limit hemorrhagic complications if there are clinical, biological, and radiological elements suggesting central nervous system vasculitis.
Patients with IBD before HSCT appeared to have a poorer prognosis after HSCT, although this difference was not statistically significant. The integrity of the intestinal epithelium is altered in NEMO deficiency-related colitis, potentially favoring bacterial translocation and increasing the risk for life-threatening systemic infections. IBD persisted in 4 patients and appeared de novo in another 2, after HSCT. These observations suggest that the pathogenesis of NEMO deficiency-related colitis may involve a nonhematopoietic component, and that HSCT does not correct IBD. This may reflect the importance of the NF-κB pathway in intestinal epithelial cells (IECs) for controlling epithelial gut integrity and interactions with the mucosal immune system and gut microflora. Indeed, in a mouse model, the inhibition of IKBKG gene expression in IECs induces the apoptosis of colonic epithelial cells, impairs antimicrobial peptide production, and favors the translocation of bacteria into the mucosa.54 This epithelial defect triggers a chronic inflammatory response in the colon, leading to severe chronic intestinal inflammation.54 Similar results were obtained when the production of other proteins of the IKK complex was disrupted, highlighting the key role of the NF-κB pathway in gut homeostasis.54,55 HSCT corrects the NEMO defect in immune cells (ie, cells derived from hematopoietic stem cells), but not in IECs, potentially accounting for the persistence of colitis after HSCT in some patients, and consistent with the late onset of colitis, after and despite HSCT. The impairment of antimicrobial peptide production in NEMO-deficient IECs may also account for the persistence of bacterial infections after HSCT in some patients. Moreover, some authors have suggested that immune reconstitution after engraftment may favor IBD because altered NEMO-deficient IECs may stimulate donor immune cells, which are responsive to TLR ligands.29 This might lead to the secretion of large amounts of TNF-α by the donor immune cells, worsening IBD. This hypothesis is supported by the observed beneficial effects of anti-TNF-α therapy in patients with postgraft colitis (P1, P7, P14, P29).
In conclusion, we show here that the overall survival of NEMO-deficient patients after HSCT is similar to that of patients with other non-SCID PIDs; pre-HSCT mycobacterial infection and IBD seem to be associated with a poorer post-HSCT prognosis; and HSCT does not appear to cure IBD. Studies of a larger cohort of patients are required to confirm these results and to compare the outcomes of patients undergoing and not undergoing HSCT, to establish consensual guidelines for the management of NEMO-deficient patients. Indeed, the decision as to whether NEMO-deficient patients should undergo HSCT is a major clinical challenge. Our results suggest the most life-threatening clinical manifestations, at least, can be cured by HSCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the sick children and their families. The authors particularly thank Luigi D. Notarangelo for his help for recruiting NEMO-deficient patients via the United States Immuno-Deficiencies network. The authors also thank Alexandra H. Filipovitch, Ashish Jain, Lizbeth Blancas Galicia, and Bobby Gaspar for their help for recruiting NEMO-deficient patients; Maya Chrabieh, Pamela Graham, and Mélanie Migaud for technical assistance; and Yelena Nemirovskaya, Eric Anderson, and Lahaouri Amar for administrative assistance.
This work was supported by the St. Giles Foundation, The Rockefeller University, INSERM, Paris Descartes University, Centre de Référence des Déficits Immunitaires Héréditaires (CEREDIH), the German Ministry for Education and Research (BMBF 01EO1303), and the National Institute for Health Research and GOSH Biomedical Research Centre. J.P.S. received a Robert A. Good/Jeffrey Modell Fellowship.
Authorship
Contribution: C.M., K.I., J.S.O., and C.P. collected, analyzed, and interpreted data and wrote the paper; C.I., A.J.M., Z.Y.K., S.D.G., T.K., R.N., E.I., I.P., S.S., J.P.S., T.O., E.C., S.C., A.O.-V., W.Q., M.Y.-N., S.E.P., A.K., N.C., S.J., J.B., A.R.G., M.A., T.G., P.V., D.M., B.N., S.B., S.M.H., G.U., B.C.-C., A.C.-N., S.E., R.D., S.Y.P., E.W.G., C.P., A.J., and T.V. provided clinical information from patients; C.M., J.R., A.P., J.B., J.-L.C., and C.P. analyzed genetic and immunological data; K.I., J.-L.C., J.S.O., and C.P. provided essential clinical information from patients, interpreted data, and wrote the paper; and C.P. designed the research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Capucine Picard, Study Center for Primary Immunodeficiency, Assistance Publique–Hopitaux de Paris, Necker Hospital for Sick Children, 156 rue de Vaugirard, 75015 Paris, France; e-mail: capucine.picard@inserm.fr.

![Figure 1. NF-kB pathway. Immune receptor signaling pathways leading to NF-κB activation can be grouped into 4 categories on the basis of the surface receptors involved: developmental receptors (receptor activator of NF-κB, vascular endothelial growth factor receptor-3, and ectodysplasin-A receptor), antigen receptors (T-cell receptor [TCR] and B-cell receptor [BCR]), members of the TNF receptor superfamily (TNF-Rs: tumor necrosis factor receptors, CD40, FAS, etc), and members of the Toll-interleukin receptors superfamily (TIR: IL-1 receptors and TLRs). The protein of the NF-kB signaling pathway (NEMO) responsible for EDA-ID is shown in black.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/12/10.1182_blood-2017-03-771600/4/m_blood771600f1.jpeg?Expires=1765991610&Signature=4UjYdkmgFacokiw3h2pcSByqK5T2o0ZXlkzvGMLsqjO7hQzBapOi5oJp~3ym-~RzFZLlXEZ26mKSwU41WmyYIviQPIPri2knnIS05umfnmhJJAKqcgZirqRoffHMq4SleBRfKLjZ04kk-R1L6TkHY8GSBWfLUxUfvVOG07OHQLOgNC1YONO9uV2jo1JHEng98G7liqeoGs7ghhz4-16~IU8ExJAqdCupnxa6KQ6uttKGeHbk1gqPNqDBvP~tFyD848Mu9hYzCEUTCPON~SkgeQhQbplP8Q8VFI7jgpR7ofTl8VTJkIbLdYxvKcjBdMC07EZy0qmtlP8QR-QLcnu5fg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal