In this issue of Blood, Eskelund et al identify a high-risk group of patients with mantle cell lymphoma (MCL) who do not experience prolonged survival despite the use of modern, cytarabine-containing induction therapy followed by autologous stem cell transplantation.1
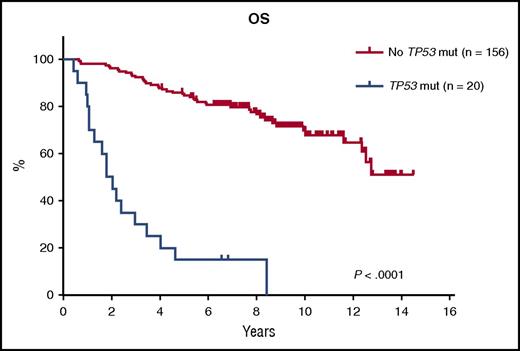
Prognostic impact of TP53 mutations on OS. Kaplan-Meier estimates of OS stratified by the presence or absence of TP53 mutations. See Figure 2D in the article by Eskelund et al that begins on page 1903.
Prognostic impact of TP53 mutations on OS. Kaplan-Meier estimates of OS stratified by the presence or absence of TP53 mutations. See Figure 2D in the article by Eskelund et al that begins on page 1903.
The researchers present an analysis based on their previously reported MCL2 and MCL3 trials, which investigated a regimen comprising rituximab, maxi-CHOP alternating with high-dose cytarabine, and consolidation with autologous stem cell transplantation (with radioimmunotherapy added prior to transplantation for responding patients in MCL3).2,3 General outcomes for these studies and other trials incorporating high-dose cytarabine and autologous stem cell transplantation have been quite promising and a remarkable improvement over prior studies. However, in their article, the authors report outcomes for patients with TP53 mutations (n = 20; median overall survival [OS] of 1.8 years) in comparison with those without TP53 mutations (n = 156; median OS not reached; see figure). A population of patients with TP53 deletions are also described, and these patients have an inferior OS as well (median, 8 years). As a result, the authors conclude that young, fit patients with TP53 aberrations are at increased risk for early progression and death, even with the receipt of aggressive therapy.
In addition to the current project, prior work has suggested that intensification of therapy does not result in prolonged survival for everyone. When the MCL International Prognostic Index is combined with the Ki-67 proliferative index (ie, MIPI-C), 11% of patients are deemed high risk, with a 5-year OS of only 17%, even after receipt of intensive induction therapies, rituximab maintenance, or both.4 Additional high-risk features associated with early progression and death include a complex karyotype (≥3 chromosomal abnormalities) and the presence of del(17p).5,6 Although each of these previously described prognostic markers have reliably identified high-risk patients, the underlying biologic cause of these features has been somewhat elusive, making selection of tailored therapy challenging.
The current article continues to shed light on the genomics of MCL and the clinical importance of mutations and genomic aberrations in this disease. This work translates prior studies that identified recurrent mutations into a clinically relevant assessment of genomic abnormalities that can be used in the risk stratification of newly diagnosed patients.7,8 The prognostic importance of TP53 mutations is certainly not a novel concept in MCL. In fact, Louie et al described a series of 23 patients with MCL over 20 years ago in which those with p53 overexpression had a median OS of only 12 months, and those without p53 overexpression had a median OS of 63 months.9 TP53 mutations and deletions have also been associated with inferior outcomes in prior series, although these series have not included a homogeneously treated population of patients who received such intensive therapy.10,11
In addition to TP53, other genomic aberrations have been associated with inferior outcomes in MCL, including NOTCH1 and CDKN2A, among others, and the recently described MCL35 proliferation signature assesses gene expression for a group of genes that are associated with proliferation and outcome in MCL.12-14 However, in this series of young, fit patients, treated with a homogeneous approach, only TP53 mutations remained a significant predictor of OS, even when accounting for currently accepted prognostic indices and other molecular aberrations. These findings would suggest that assessing for TP53 aberrations should be considered a priority when only a limited panel of molecular assessments is feasible.
As we are improving our ability to identify the highest-risk patients, including those who do not respond to our most aggressive treatments, the question of how to best manage these patients remains unanswered. Not offering aggressive therapy to otherwise suitable candidates does not seem appropriate in the absence of a viable alternative. The answer will most likely reside with novel approaches to therapy. The combination of rituximab-lenalidomide, for example, has demonstrated excellent efficacy in a single-arm phase 2 study with no association of MIPI or Ki67 with response rate or progression-free survival.15 However, patients with a high-risk MIPI score still had inferior OS in this study, and the presence of genomic aberrations has not been reported. Additional novel therapies such as ibrutinib have been largely evaluated in the relapsed setting, but Wang et al have presented findings combining rituximab with ibrutinib in untreated patients, with an overall response rate of 100%, suggesting that baseline prognostic markers may not have an impact on the benefit of this therapy.16 However, further follow-up is needed, and the assessment of baseline mutation status has not been reported for most upfront therapies. As a result, the impact of TP53 and other mutations on outcomes for patients receiving novel therapies remains unclear.
This article contributes to the growing body of literature suggesting that currently available, aggressive induction therapies for MCL are highly effective for most, but not all, patients with MCL. Improved identification of these high-risk patients is important and will contribute to our ability to tailor therapies for them. Given the experiences seen in chronic lymphocytic leukemia and acute myelogenous leukemia, it is not surprising that simply combining high-dose chemotherapy does not result in prolonged remission for the highest-risk patients. We will need novel approaches and combinations to successfully manage this group of patients, and this remains a critical unmet medical need.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal