In this issue of Blood, Zhao et al use a humanized mouse model to investigate the mechanisms driving daily oscillations in circulating human and murine leukocytes.1 In the same mice, they find human and murine circulating leukocytes displaying inverted oscillations, reproducing the trafficking pattern previously observed in both species. A novel network regulating circadian leukocyte trafficking is proposed. It involves interspecies differences of stress-kinase regulation of reactive oxygen species (ROS), hypoxia-inducible factor 1α (HIF-1α) and clock gene–dependent regulation of the CXCL12 receptor CXCR4. This study underscores the crosstalk of the genetic clock with metabolism and ROS in the regulation of leukocyte migration and reveals new mechanistic players.
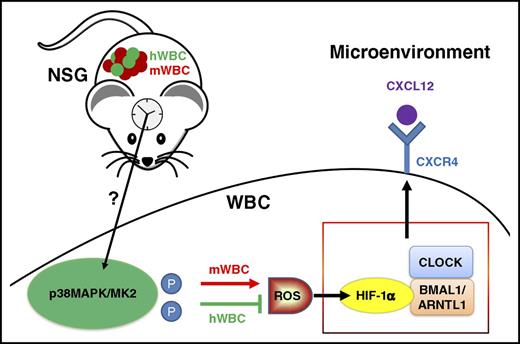
Model proposed in the study by Zhao et al. Physiological activation of p38MAPK/MK2 during circadian cycles causes differential ROS levels and HIF-1α–BMAL1-dependent CXCR4 regulation, explaining opposite trafficking patterns of mouse and human leukocytes in chimeric immunodeficient mice. hWBC, human white blood cell; mWBC, mouse white blood cell; P, phosphorylation; WBC, white blood cell.
Model proposed in the study by Zhao et al. Physiological activation of p38MAPK/MK2 during circadian cycles causes differential ROS levels and HIF-1α–BMAL1-dependent CXCR4 regulation, explaining opposite trafficking patterns of mouse and human leukocytes in chimeric immunodeficient mice. hWBC, human white blood cell; mWBC, mouse white blood cell; P, phosphorylation; WBC, white blood cell.
Circadian rhythms allow for the organism’s adjustment to basic day/night changes, such as activity/sleep or feeding cycles. These are governed at the organismal level by the pacemaker in the brain, the suprachiasmatic nucleus, which receives light input through the retinohypothalamic tract and synchronizes peripheral organs via the autonomic nervous system and the hypothalamic-pituitary-adrenal axis on a daily basis. At the cellular level, peripheral oscillators exist in many cell types and regulate metabolism, proliferation, and function. Different clocks interact with each other to ensure robust responses. Core clock genes, such as BMAL1/ARNTL1, regulate the transcription of multiple genes, including other clock genes that drive transcription-translation loops over 24 hours (reviewed in Curtis et al2 and Takahashi3 ).
Oscillations previously found both in the number and the activity of hematopoietic progenitors and leukocytes might have important implications for regeneration and response to infections.4-6 For instance, oscillations of Bmal1 expression in inflammatory monocytes regulate chemokine genes and immune response.7 Inverted oscillations in circulating leukocytes have been previously reported in (nocturnal) mice and (diurnal) humans.4-6 In both species, leukocytes are preferentially released from the bone marrow into circulation during the resting period. However, the mechanisms explaining interspecies differences in leukocyte trafficking have remained elusive.
Zhao et al create hematopoietic chimeric mice to study the trafficking of human and murine leukocytes. The model consists of neonatal NOD-SCID IL-2Rγ−/− (NSG) mice sublethally irradiated and intrahepatically transplanted with CD34+ human fetal liver cells. Then, 8- to 12-week-old mice carrying 30% to 50% human CD45+ cells are selected for circadian studies.
Strikingly, the same chimeric mice show inverted trafficking patterns for human and murine leukocytes, reproducing the interspecies differences. Previous studies in mice and humans4,5 have shown oscillations of the CXCL12-CXCR4 signaling pathway, a key regulator of leukocyte migration. In C57BL/6 mice, previous studies have shown oscillations in bone marrow Cxcl12 expression.4,8 Zhao et al do not find obvious Cxcl12 messenger RNA oscillations in NSG mice. However, because the number of experimental mice studied was lower, the sampling less frequent, and the time points performed at other times, it remains unclear whether there are differences from the strain/immunodeficiency and/or the transplant setting or not. A similar consideration applies to BMAL1 messenger RNA expression, which does not seem to oscillate in mouse or human leukocytes in the chimeric mice, but has been previously shown to oscillate in leukocyte subsets.7
Regardless, the sharp difference of mouse/human leukocyte trafficking in the same environment argues for key cell-autonomous mechanisms. Consistent with previous studies,5,8 they find oscillations in CXCR4 expression in antiphase with circulating leukocytes. Blockade of Cxcl12 (which is murine derived in the humanized model) blunts oscillations of both murine and human leukocytes. Blockade of the human receptor has similar consequences on the human leukocytes, pointing toward a major role of CXCR4. To understand how the murine and human receptors are differentially regulated during circadian cycles, the authors profile clock gene expression in leukocytes. Intriguingly, in the humanized model, the peripheral oscillator appears to be present in mouse leukocytes, but not in human leukocytes. This interesting difference points toward species-specific regulation of CXCR4 (and possibly other adhesion receptors) in relation to the genetic clock, leaving a fertile area for future studies.
The authors find that CXCR4 oscillations are abolished in BMAL1-deficient leukocytes, consistent with previous findings in mice.5 Therefore, they hypothesize that a clock network–independent BMAL1 function regulates CXCR4. Because HIF-1α expression follows circadian oscillations, regulates CXCR4, and binds BMAL1, the authors measure HIF-1α expression and find direct correlations with CXCR4 expression. Because HIF-1α is regulated by ROS, they measure ROS levels in human and murine leukocytes and find inverted expression patterns during circadian cycles. Treatment with the ROS inhibitor N-acetylcysteine abrogated oscillations in HIF-1α, CXCR4, and circulating leukocytes. They then hypothesize that the p38MAPK/MK2 pathway might generate opposite ROS levels in murine and human leukocytes. Indeed, the authors show different p38 phosphorylation levels at distinct circadian time points and elegantly demonstrate that MK2 inhibition abolishes circadian oscillations of ROS, and the downstream pathways. Notably, MK2 inhibition increases ROS in murine leukocytes, whereas it has the opposite effect on the human cells, pointing toward a key pathway responsible for the interspecies difference.
Finally, the authors examine the possible connection of this pathway with the central pacemaker using a jet lag experimental setting. Shifting the light cycles abrogates oscillations not only of leukocytes, but also of p38/MK2 phosphorylation, suggesting that the suprachiasmatic nucleus might be able to regulate stress kinases in leukocytes (see figure).
In summary, the detailed study by Zhao et al expands previous findings on the regulation of the CXLC12-CXCR4 axis by the genetic clock4,5 to interactions with stress kinases and ROS. Other studies have found clock gene–independent ROS-related mechanisms that maintain circadian cycles. For instance, ROS-regulating enzymes, such as peroxiredoxins, are ancient clocks that have been conserved throughout evolution.9 In Arabidopsis, the redox rhythm interacts with the genetic clock to adjust the plant’s response to environmental stimuli and pathogens.10 Studies along these lines will help determine the fine crosstalk between metabolic and genetic clocks. This knowledge will likely affect the successfulness of “chronotherapy” approaches.
Conflict-of-interest disclosure: S.M.-F. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal