In this issue of Blood, Issa et al1 report that patients with chronic-phase chronic myeloid leukemia (CML) in remission after treatment with tyrosine kinase inhibitors (TKIs) with clonal chromosomal aberrations (CCAs) in Philadelphia chromosome–negative (Ph−) metaphases had a significantly worse survival than similar patients without CCAs. This study is notable for the large number of patients with CCAs (n = 58) and the length of follow up (median, 7.6 years) (see figure).
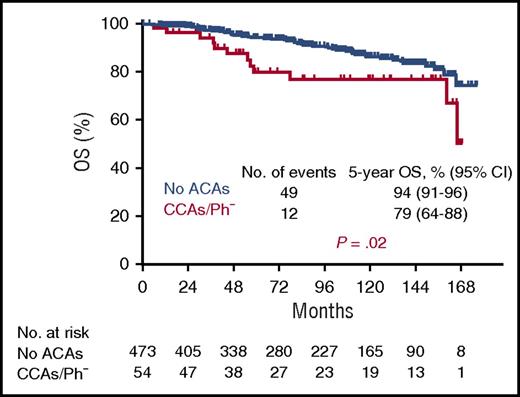
Overall survival (OS) of 54 patients with CML with CCAs/Ph− compared with 473 patients with no additional chromosomal aberrations (ACAs). Median observation time was 7.6 years. See supplemental Figure 1D in the article by Issa et al that begins on page 2084.
Overall survival (OS) of 54 patients with CML with CCAs/Ph− compared with 473 patients with no additional chromosomal aberrations (ACAs). Median observation time was 7.6 years. See supplemental Figure 1D in the article by Issa et al that begins on page 2084.
Fifteen years after the initial report of CCAs in Ph− metaphases in imatinib responders with CML,2 the report by Issa et al addresses the question of the prognostic relevance of CCAs/Ph−. Up to now, most studies have included only a few patients, with limited observation time, and have not provided convincing prognostic data.2-6 The frequency of transition to myelodysplastic syndromes (MDSs) have also remained unclear, as has the impact of age on CCAs. The study by Issa et al is the first with sufficient patients and long enough follow-up to address these questions.
Although the 58 patients with CCAs/Ph− were 12 years older than patients without ACAs, presence of CCAs/Ph− remained a significant independent prognostic factor in multivariate analysis. In 23 patients, loss of the Y chromosome (−Y) was the only abnormality, which has uncertain prognostic significance. The remaining 35 patients with CCAs/Ph− were analyzed separately and were found to have a worse prognosis.
What accounts for this inferior prognosis? The incidence of transformation to advanced phases of CML was similar to that among patients with ACAs in Ph+ cells. This finding was due to inclusion of early response in the multivariate analysis (<10% BCR-ABL1:ABL1 ratio after 3 months); exclusion of patients not meeting the 3-month milestone negated the adverse impact of CCAs/Ph− on prognosis.
CCAs/Ph− were observed in ∼10% of 598 patients with Ph+ CML in chronic phase. This is a higher percentage than reported in an earlier 2004 series of 1001 patients, of whom only 34 (3.4%) had CCAs/Ph−.3 The reason for the higher incidence of CCAs/Ph− might be more systematic cytogenetic analyses in the study by Issa et al. The type of TKI did not seem to play a role. The incidence of ACAs in Ph− metaphases (clonal and nonclonal) was similar in patients receiving imatinib (16%) and dasatinib (14%), with a slightly higher incidence in those receiving nilotinib (25%) and ponatinib (22%). The most commonly detected CCAs/Ph− were −Y in 25 patients, trisomy 8 in 7 patients, and complex CCAs/Ph− and monosomy 7 in 4 patients each. This is not much different from the data reported by Terre et al,3 who found trisomy 8 in 12 patients, monosomy 7 in 7 patients, and −Y and other deletions in 5 patients each. Trisomy 8 and monosomy 7 are the aberrations most frequently reported by others.4-6 Not all CCAs/Ph− were equally adverse in their effects; −7 and complex (≥3) aberrations carried the worst prognosis, whereas trisomy 8 as the only aberration had a prognosis similar to that of no ACAs.
Only 2 patients, both with monosomy 7, experienced progression to MDSs or acute myeloid leukemia, demonstrating that progression of patients with CCAs/Ph− to MDSs is a rare event in the absence of monosomy 7. A third patient with monosomy 7 died as a result of blast crisis. Dysplastic features, mostly cytopenias and macrocytosis, as described in early reports, could in part also be explained as treatment effects during a prolonged recovery phase of normal hematopoiesis. The biologic determinants of why −7 is associated with a risk of developing MDSs should be the subject of additional studies.
The major reason for decreased survival in patients with CCAs/Ph− seems to be transformation of CML resulting from genetic instability and the appearance of ACAs, similar to observations in Ph+ cells.7,8 The occurrence of ACAs in Ph+ and Ph− cells in the same patient, as observed by others as well,9,10 would support this idea. Clonal ACAs in CML constitute a negative prognostic sign whether they occur in Ph+ or Ph− cells.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal