In this issue of Blood, Dubert et al present the results of a large cohort study conducted across 3 sub-Saharan African countries (Mali, Cameroon, and Ivory Coast) to quantify differences between subphenotypes of sickle cell disease (SCD) based on markers of anemia, hemolysis, and vascular complications.1
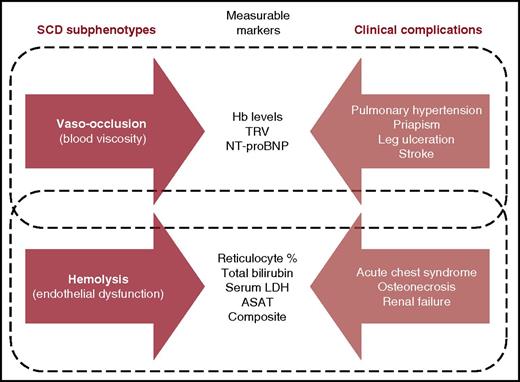
Subphenotypes of SCD, measurable markers, and clinical complications. ASAT, aspartate aminotransferase; Hb, hemoglobin; LDH, lactate dehydrogenase; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; TRV, tricuspid regurgitant jet velocity. Adapted from Figure 2 in Kato et al.7
Subphenotypes of SCD, measurable markers, and clinical complications. ASAT, aspartate aminotransferase; Hb, hemoglobin; LDH, lactate dehydrogenase; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; TRV, tricuspid regurgitant jet velocity. Adapted from Figure 2 in Kato et al.7
There is growing recognition that SCD represents an increasing global health burden.2 This is partly illustrated by recent efforts of the American Society of Hematology, including the Conquer SCD initiative, to raise awareness about the disease. Although precise estimates of the global number of patients with SCD are currently lacking, it is clear that the vast majority of this burden occurs in sub-Saharan Africa. The total population of ∼100 000 with SCD in the United States3 is very small in comparison with the estimated 260 000 children who are born with sickle cell anemia (SCA) across sub-Saharan Africa every year.2
Although remarkable progress has been made toward a better understanding and improved survival of patients with SCD in the United States and other high-income countries, advances in Africa have been more limited. Furthermore, important differences exist in the frequency of a range of clinical complications observed in patients with SCD between high-income and African countries. Leg ulcers, for example, are much more common in low- and middle-income counties (LMICs) than in the United States or United Kingdom. The “Cœur, Artères et DREpanocytose” (CADRE) cohort is a large multinational prospective study of patients with SCD from 5 countries across West and Central Africa, although data from only 3 of these countries (Mali, Cameroon, and Ivory Coast) were included in the current study. Cohorts like CADRE have the potential to considerably improve our understanding of chronic complications, including cardiovascular events, and of the mechanisms underlying the vascular complications of SCD in LMICs. Identifying reliable “universal” predictors of clinical complications and disease severity is essential to define sustainable prevention and management policies.4
The relative roles of hemolysis and vaso-occlusion in the pathophysiology of SCD is a subject of some controversy.5 The hyperhemolysis paradigm (HHP), through which it is hypothesized that chronic hemolysis in SCD sequentially causes increased cell-free plasma hemoglobin, nitric oxide biodeficiency, endothelial dysfunction, pulmonary hypertension, and vascular complications, led to the concept of 2 different subphenotypes of SCD: one characterized by the constellation of high hemoglobin levels, vaso-occlusive pain crises, acute chest syndrome, and osteonecrosis and the other by hemolysis, pulmonary hypertension, priapism, leg ulcers, and stroke (see figure).6,7 The controversy involves both the concept that there might be a clear divide between these 2 subphenotypes and the extent of the overlap between them. Furthermore, studies supporting the HHP have mostly been conducted in the United States, and to date, its appraisal in the context of SCD in Africa has been extremely limited. By studying the associations between markers of anemia, hemolysis, and vascular complications within the CADRE cohort, Dubert et al provide the first large-scale study exploring the HPP controversy in an African setting. The authors found that within CADRE, severe anemia was associated with elevated tricuspid regurgitant jet velocity, microalbuminuria, and leg ulcers, but these vascular complications were not independently associated with indirect markers of increased hemolysis.
No hypothesis can be overturned by a single study, and the full picture regarding the validity of the HHP will require further work by multiple investigators. As acknowledged by the authors, their study has a number of caveats. For a start, within individuals, hemolysis is not necessarily a stable phenotype, and relating outcomes to an assessment made at a single time point will always therefore be questionable. Moreover, the authors’ “hemolysis index” was different from that used by other investigators and was not ground-truthed against direct measures. The wide age range and geographic spread of their cohort also brings analytical challenges. Furthermore, an important omission in this study was the lack of data on α-thalassemia, an important modifier of SCD that might potentially shift patients from one subphenotype to the other.7 Nevertheless, the authors set out to investigate the HHP with an open mind and found only limited support for a clear phenotypic divide based on the assessment of hemolysis alone. This study illustrates some of the real difficulties associated with the collection of rich phenotype data under challenging circumstances and the remarkable levels of morbidity that are seen in Africa, particularly within adult populations.
Beyond the HHP controversy, Dubert et al’s study also documents 2 additional challenges concerning the prevention and treatment of SCD in Africa. First, the authors provide clear evidence for significant differences between some of the different genotypes of SCD, including SCA, sickle-β0 thalassemia, sickle-β+ thalassemia, and hemoglobin SCD in terms of baseline characteristics and clinical complications. Although this observation is not in itself novel, the size of this study reinforces the potential need for the tailored treatment of different SCD subgroups. Second, this study highlights the need for reliable easy-to-use markers of anemia and hemolysis in LMICs. The etiology of anemia in African patients with SCD may be very different from that in resource-rich countries, potentially including additional factors such as malnutrition (eg, iron deficiency) and infectious diseases (eg, malaria and helminth infestations). Accurately quantifying hemolysis requires direct measurements of the lifespan of red blood cells. Most studies published in relation to the HHP so far, including this one, have used indirect markers such as reticulocyte counts, aspartate aminotransferase, total bilirubin, and lactate dehydrogenase levels. Technological advances similar to those seen in recent years in the development of point-of-care testing devices8 would be a valuable asset to this research agenda going forward.
Assuming that financial and logistical challenges can be overcome, the sheer number of patients affected by SCD in both sub-Saharan Africa and India offers a huge opportunity to further our understanding of the epidemiology and pathophysiology of this important condition. Better data will help us to define the optimum approaches to prevention and management policies in LMICs and reduce the global burden of SCD. Further work of the type described in this article will help us to piece together the pathophysiology of SCD for the benefits of all patients with this condition, wherever they may live.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal