Abstract
Patients with sickle cell disease (SCD) suffer from intense pain that can start during infancy and increase in severity throughout life, leading to hospitalization and poor quality of life. A unique feature of SCD is vaso-occlusive crises (VOCs) characterized by episodic, recurrent, and unpredictable episodes of acute pain. Microvascular obstruction during a VOC leads to impaired oxygen supply to the periphery and ischemia reperfusion injury, inflammation, oxidative stress, and endothelial dysfunction, all of which may perpetuate a noxious microenvironment leading to pain. In addition to episodic acute pain, patients with SCD also report chronic pain. Current treatment of moderate to severe pain in SCD is mostly reliant upon opioids; however, long-term use of opioids is associated with multiple side effects. This review presents up-to-date developments in our understanding of the pathobiology of pain in SCD. To help focus future research efforts, major gaps in knowledge are identified regarding how sickle pathobiology evokes pain, pathways specific to chronic and acute sickle pain, perception-based targets of “top-down” mechanisms originating from the brain and neuromodulation, and how pain affects the sickle microenvironment and pathophysiology. This review also describes mechanism-based targets that may help develop novel therapeutic and/or preventive strategies to ameliorate pain in SCD.
Introduction
A hallmark of sickle cell disease (SCD) is intense pain that can start during infancy and increase in severity throughout life, leading to hospitalization and poor quality of life.1-3 SCD is an autosomal recessive disorder caused by substitution of a valine residue for glutamic acid at position 6 in β-globin, resulting in sickle hemoglobin (HbS).4 A unique feature of SCD is vaso-occlusive crises (VOCs) characterized by episodic, recurrent, and unpredictable episodes of acute pain.1-3 Microvascular obstruction during a VOC leads to impaired oxygen supply to the periphery and ischemia-reperfusion injury, inflammation, oxidative stress, and endothelial dysfunction, all of which may perpetuate a noxious microenvironment leading to pain.1-9 Although several recent reviews provide an up-to-date understanding of clinical features, epidemiology, and underlying pathobiology of SCD,5-9 the understanding of underlying mechanisms of pain remains a critical unmet need.
Here, we describe the current understanding of cellular and molecular mechanisms of pain in SCD. Concurrently, a brief overview of neuronal and molecular machinery underlying pain processing is presented to help targeting of nociceptive mechanisms in SCD (see Table 1 for definitions of nociception-related terminology). Because current treatment of moderate to severe pain is mostly reliant upon opioids, we highlight mechanism-based targets underlying the side effects of opioids specific to SCD, with the goal of developing strategies to optimize opioid analgesia without inadvertent side effects.
Definition of key terms
| Action potential: Rise and fall in the membrane potential of a cell leading to the transmission of electrical impulses along neurons |
| Allodynia: Pain evoked by painless stimuli |
| Analgesia: Lack of pain upon painful stimulus |
| Antidromic impulse: An impulse traveling in the opposite direction of regular action potential (i.e., from the central nervous system to the periphery), contributing to the release of neuroinflammatory mediators and peripheral sensitization |
| Ascending pathway: A neural circuit that carries sensory and nociceptive signals from peripheral receptors to the central nervous system |
| A-β fibers: Thickly myelinated fibers conducting the sensation of touch, vibration, and proprioception; they are referred to as fast-conducting fibers with a speed of ∼35-120 meters/second |
| A-δ fibers: Thinly myelinated fibers associated with acute pain and sensation of cold and pressure; their conduction velocity of action potentials is 5–30 meters/second |
| Axon reflex: Movement of action potentials in C-fibers peripherally releasing vasoactive and inflammatory substances such substance P |
| C-fibers: Unmyelinated fibers responsive to a wide range of noxious stimuli including, mechanical, thermal, and pathophysiologic factors; these fibers are involved in a slow but long-lasting painful sensation of burning. Their conduction velocity of action potentials is 0.5–2.0 meters/second |
| Central sensitization: Increased responsiveness of second-order neurons to subthreshold inputs after a sustained noxious stimulus |
| Hyperalgesia: An increased painful sensation to a noxious stimulus |
| Hyperesthesia: Heightened sensitivity to painless and painful stimuli |
| Neurogenic inflammation: Inflammation caused by the release of inflammatory mediators from peripheral nerve fibers after stimulation by a noxious stimulus |
| Nociceptors: Receptors in the peripheral somatosensory nervous system involved in the detection and transduction of noxious stimuli |
| Noxious stimulus: A stimulus that could cause tissue damage; it can be mechanical, chemical, or thermal |
| Pain: An unpleasant sensory and emotional experience caused by injury or pathophysiology |
| Pain threshold: Lowest intensity of stimulus that evokes pain |
| Paresthesia: A sensation that is abnormal |
| Peripheral sensitization: Increased response to noxious stimuli at the site of injury resulting from the decreased threshold for depolarization in the peripheral nerve fiber nociceptors |
| Tolerance: Decreased response to a substance after long-term use, requiring a dosage increase to accomplish similar effects |
| Action potential: Rise and fall in the membrane potential of a cell leading to the transmission of electrical impulses along neurons |
| Allodynia: Pain evoked by painless stimuli |
| Analgesia: Lack of pain upon painful stimulus |
| Antidromic impulse: An impulse traveling in the opposite direction of regular action potential (i.e., from the central nervous system to the periphery), contributing to the release of neuroinflammatory mediators and peripheral sensitization |
| Ascending pathway: A neural circuit that carries sensory and nociceptive signals from peripheral receptors to the central nervous system |
| A-β fibers: Thickly myelinated fibers conducting the sensation of touch, vibration, and proprioception; they are referred to as fast-conducting fibers with a speed of ∼35-120 meters/second |
| A-δ fibers: Thinly myelinated fibers associated with acute pain and sensation of cold and pressure; their conduction velocity of action potentials is 5–30 meters/second |
| Axon reflex: Movement of action potentials in C-fibers peripherally releasing vasoactive and inflammatory substances such substance P |
| C-fibers: Unmyelinated fibers responsive to a wide range of noxious stimuli including, mechanical, thermal, and pathophysiologic factors; these fibers are involved in a slow but long-lasting painful sensation of burning. Their conduction velocity of action potentials is 0.5–2.0 meters/second |
| Central sensitization: Increased responsiveness of second-order neurons to subthreshold inputs after a sustained noxious stimulus |
| Hyperalgesia: An increased painful sensation to a noxious stimulus |
| Hyperesthesia: Heightened sensitivity to painless and painful stimuli |
| Neurogenic inflammation: Inflammation caused by the release of inflammatory mediators from peripheral nerve fibers after stimulation by a noxious stimulus |
| Nociceptors: Receptors in the peripheral somatosensory nervous system involved in the detection and transduction of noxious stimuli |
| Noxious stimulus: A stimulus that could cause tissue damage; it can be mechanical, chemical, or thermal |
| Pain: An unpleasant sensory and emotional experience caused by injury or pathophysiology |
| Pain threshold: Lowest intensity of stimulus that evokes pain |
| Paresthesia: A sensation that is abnormal |
| Peripheral sensitization: Increased response to noxious stimuli at the site of injury resulting from the decreased threshold for depolarization in the peripheral nerve fiber nociceptors |
| Tolerance: Decreased response to a substance after long-term use, requiring a dosage increase to accomplish similar effects |
Pain in SCD
The median survival of patients with SCD in the 1970s was estimated to be 20 years, but with the implementation of antibiotic prophylaxis against encapsulated organisms, vaccinations, hydroxyurea, and improved management, patients are surviving into their 50s and 60s in the Western world.10-12 However, pain remains a major consequence of SCD. Clinically, pain in SCD is experienced as either acute episodes or as chronic ongoing pain.1,13 The complex pathobiology of SCD suggests that pain may occur via nociceptive, inflammatory, and neuropathic mechanisms.1 Each pain state generates its own neurochemical signatures in the periphery and the central nervous system (CNS) and requires different analgesic treatment.14 On the basis of clinical observations and transgenic sickle mice, pain can be spontaneous as well as evoked, as described below.
Humanized mouse models of SCD with clinically relevant features of sickle pain
Transgenic HbSS-BERK and HbSS-Townes mice expressing human sickle hemoglobin exhibit the characteristic features of pain and have relatively high opioid analgesic requirements, similar to that observed in patients with SCD.3,15-18 Pain behavior measures of HbSS-BERK/Townes mice show increased mechanical and thermal hyperalgesia (cold and heat) and deep/musculoskeletal pain (Table 2). This increased sensitivity in mice is analogous to the increased sensitivity to touch and temperature extremes and multifocal musculoskeletal pain in patients with SCD.19 Consequent to sensory testing in sickle mice (Table 2), quantitative sensory testing has demonstrated increased sensitivity to mechanical and thermal stimuli in patients with SCD.15-17,19-24 Furthermore, we have been able to evoke acute pain in these mice with hypoxia/reoxygenation (H/R) treatment to simulate a VOC.16 Finally, neurochemical signatures of pain in sickle mice parallel those observed in patients with SCD.15,25-31 These sickle mice have thus provided critical insights into the mechanisms of sickle pain, with potential for clinical translation.
Pain behavior analysis in transgenic mice with sickle cell disease
| Pain characteristic . | Device . | Response . | Sensory stimuli . |
|---|---|---|---|
| Thermal (heat) | Hargreaves’ apparatus | Paw withdrawal latency | Heat targeted to the plantar surface of the hind paw |
| Thermal (cold) | Cold plate | Paw withdrawal latency and frequency | Cold at 4°C |
| Mechanical | Von Frey monofilaments | Paw withdrawal frequency | Application of filament to the plantar surface of the hind paw |
| Deep tissue/musculoskeletal | Grip force meter | Grip force exerted | None |
| Facial expression | Mouse grimace scale | Orbital tightening, ear position, nose bulge, cheek bulge | None |
| Conditioned place preference | Conditioned place preference boxes | Time spent in specific chamber | None |
| Vocalization after-discharge | Pressure-zone microphone | Vocalization | None |
| Pain characteristic . | Device . | Response . | Sensory stimuli . |
|---|---|---|---|
| Thermal (heat) | Hargreaves’ apparatus | Paw withdrawal latency | Heat targeted to the plantar surface of the hind paw |
| Thermal (cold) | Cold plate | Paw withdrawal latency and frequency | Cold at 4°C |
| Mechanical | Von Frey monofilaments | Paw withdrawal frequency | Application of filament to the plantar surface of the hind paw |
| Deep tissue/musculoskeletal | Grip force meter | Grip force exerted | None |
| Facial expression | Mouse grimace scale | Orbital tightening, ear position, nose bulge, cheek bulge | None |
| Conditioned place preference | Conditioned place preference boxes | Time spent in specific chamber | None |
| Vocalization after-discharge | Pressure-zone microphone | Vocalization | None |
Somatosensory system and generation of pain
The neurobiology of pain is known to involve the transduction, transmission, modulation, and perception of pain via somatosensory and limbic systems (Figure 1).32-34 The first step is the conversion of a noxious stimulus (including mechanical, chemical, thermal, and other algogenic agents) into an electrical nerve impulse in the form of an action potential. An action potential is generated by an influx of positively charged ions into an axon through membrane voltage-gated ion channels. Action potentials propagate through the primary afferent nerve fibers whose cell bodies in the spinal cord dorsal root ganglion (DRG) synapse with second-order neurons in the dorsal horn of the spinal cord (Figure 1). Second-order neurons are responsive to noxious inputs from A-δ and C-fibers, and non-noxious inputs from A-β fibers. A-δ fibers respond to mechanical and thermal stimuli and are responsible for sharp, acute pain, such as that perhaps evoked by a VOC. These fibers activate second-order neurons instantaneously. C-fibers respond to a broad range of stimuli, including thermal, mechanical, and chemical stimuli producing slow-burning and long-lasting dull pain, and activate second-order neurons by sustaining the depolarized state for a long period of time. A-β fibers carry touch sensation; the activation of these fibers can suppress pain (such as massaging a painful area to relieve pain).
Sickle cell disease from a point mutation to a systemic dysfunction and pain. Circulation of sickle RBCs leads to multiple pathophysiologic problems, including, but not limited to, hemolysis, hypoxia/reperfusion, ischemia, excessive inflammation and free heme, vascular dysfunction, organ damage, and vaso-occlusion (left panel). Each of these can contribute to a noxious microenvironment evoking nociceptive mechanisms of pain. Emerging data have identified several cellular and molecular targets that contribute to nociceptive activity in sickle cell disease (middle panel). These targets may be located in the periphery and/or the CNS, suggesting that the sickle microenvironment can activate the transmission of pain from the periphery as well as influence the CNS directly. Mechanisms of nociception are complex, involving the peripheral neural activity and CNS (right panel) involving several different processes. (1) Transduction involves the generation of action potentials (electrical activity) from the noxious environment in the periphery; (2) transmission is the process of transmitting the action potentials to the dorsal horn of the spinal cord through the primary afferents and first-order neurons in the DRG; (3) modulation is the complex processing of signals in the dorsal horn of the spinal cord after activation of second-order neurons and neuromodulation (amplification or inhibition) from interneurons and/or descending projections from the brainstem with inhibitory or facilitatory pathways involving neurotransmitters; this processing of neural activity results in the inhibition or facilitation of nociceptive activity, which is relayed to the higher brain centers; and (4) perception is the transcription of nociceptive signals to the subjective emotional experience of pain in the higher centers of brain. There are exceptions, including “top down” mechanisms of pain and perception-based modulation as described in the text. In addition, peripheral and/or central sensitization may occur in response to ongoing noxious stimuli, resulting in reduced firing threshold potential leading to the generation of pain with innocuous stimuli. Nerve impulses travel orthodromically from the periphery to the spinal cord, but under sustained activation they can travel antidromically (dashed brown arrow), releasing neurotransmitters such as SP in the periphery. In addition, release of neuropeptides can also occur in the periphery from activated axonal nerve endings by axonal reflex. Due to the genetic nature of SCD, an ongoing noxious microenvironment replete with algogenic factors may induce the nociceptive mechanisms during infancy and sustain the activation through adulthood if the disease remains uncontrolled, leading to peripheral and central sensitization resulting in chronic pain recalcitrant to therapy. ER stress, endoplasmic reticulum stress; Glu, glutamic acid; Pro, proline; sRBC, sickle red blood cell; TLR4, Toll-like receptor 4; Val, valine.
Sickle cell disease from a point mutation to a systemic dysfunction and pain. Circulation of sickle RBCs leads to multiple pathophysiologic problems, including, but not limited to, hemolysis, hypoxia/reperfusion, ischemia, excessive inflammation and free heme, vascular dysfunction, organ damage, and vaso-occlusion (left panel). Each of these can contribute to a noxious microenvironment evoking nociceptive mechanisms of pain. Emerging data have identified several cellular and molecular targets that contribute to nociceptive activity in sickle cell disease (middle panel). These targets may be located in the periphery and/or the CNS, suggesting that the sickle microenvironment can activate the transmission of pain from the periphery as well as influence the CNS directly. Mechanisms of nociception are complex, involving the peripheral neural activity and CNS (right panel) involving several different processes. (1) Transduction involves the generation of action potentials (electrical activity) from the noxious environment in the periphery; (2) transmission is the process of transmitting the action potentials to the dorsal horn of the spinal cord through the primary afferents and first-order neurons in the DRG; (3) modulation is the complex processing of signals in the dorsal horn of the spinal cord after activation of second-order neurons and neuromodulation (amplification or inhibition) from interneurons and/or descending projections from the brainstem with inhibitory or facilitatory pathways involving neurotransmitters; this processing of neural activity results in the inhibition or facilitation of nociceptive activity, which is relayed to the higher brain centers; and (4) perception is the transcription of nociceptive signals to the subjective emotional experience of pain in the higher centers of brain. There are exceptions, including “top down” mechanisms of pain and perception-based modulation as described in the text. In addition, peripheral and/or central sensitization may occur in response to ongoing noxious stimuli, resulting in reduced firing threshold potential leading to the generation of pain with innocuous stimuli. Nerve impulses travel orthodromically from the periphery to the spinal cord, but under sustained activation they can travel antidromically (dashed brown arrow), releasing neurotransmitters such as SP in the periphery. In addition, release of neuropeptides can also occur in the periphery from activated axonal nerve endings by axonal reflex. Due to the genetic nature of SCD, an ongoing noxious microenvironment replete with algogenic factors may induce the nociceptive mechanisms during infancy and sustain the activation through adulthood if the disease remains uncontrolled, leading to peripheral and central sensitization resulting in chronic pain recalcitrant to therapy. ER stress, endoplasmic reticulum stress; Glu, glutamic acid; Pro, proline; sRBC, sickle red blood cell; TLR4, Toll-like receptor 4; Val, valine.
Second-order dorsal horn neurons allow the transmission of either pain or touch sensation to the brain. These neurons are activated by glutamate and substance P (SP). The spinal dorsal horn acts as a “gate” for the convergence of fibers from the periphery and descending fibers from higher brain centers. Impulses transmitted from the periphery to this locus may be modulated by inputs from the descending neuronal fibers as well as large A-β fibers, following a complex interplay with excitatory and inhibitory neurotransmitters and interneurons. Collectively, these interactions are referred to as the “gate control theory.”
The descending pain-modulating system originating from higher centers of the brain plays a critical role in the perception of pain by inhibiting or amplifying nociceptive inputs through the neurotransmitters serotonin, dopamine, endogenous opioids, γ-amino butyric acid, glycine, and others. Modulated signals are processed in brain areas responsible for sensory perception and for elicitation of affect, emotion, and memory. The nature and severity of conscious pain perception depend on the complex processing in different brain areas, including the somatosensory cortex, the prefrontal cortex, the amygdala, and the anterior cingulate cortex. Recently, attention has been focused on perception-based components of pain because of their ability to reduce pain via nonpharmacologic behavioral techniques, such as guided imagery, mindfulness and relaxation training, hypnosis, and cognitive behavioral therapy.35-37
Chronic pain and central sensitization in SCD
Chronic pain occurs in a significant proportion of patients with SCD and increases with age.19,38 If pain lasts for >3 months, it is considered chronic according to the Expert Panel Report, 2014.39 The Pain in Sickle Cell Epidemiology Study (PiSCES) showed that chronic pain occurred in 55% of adults at home on more than half of the days and in 29% of adults on 95% of days.13 The biopsychosocial mechanisms and classifiers of chronic pain conditions in SCD are described in a recent comprehensive report of the collaborative working group representing several organizations associated with pain and addiction, the US Food and Drug Administration, and the American Pain Society to develop the American Pain Society Taxonomy.40
The etiology of chronic pain in SCD is not clear. However, causes underlying chronic pain include extended hyperalgesia after a VOC, organ-specific pain such as avascular necrosis, and opioid-induced hyperalgesia.39,40 Oxycodone and codeine were the most frequently used analgesics in a multicenter study of hydroxyurea in 299 patients.41 The PiSCES project showed that opioids were used during a majority of pain days (78%), with long- and short-acting opioids used by 38.8% and 47% patients, respectively.42 An understanding of the possible mechanisms underlying chronic pain described below may lead to opioid-reducing treatment strategies.
Central sensitization
Chronic sickle pain may be a distinct pathophysiologic entity because the initial origin of injury may not be relevant once sensory pathways shift to a state of hyperexcitability. This process involves peripheral nociceptor sensitization via the activation of ion channels, including transient receptor potential vanilloid 1 (TRPV1) and sodium channels, and impairment of inhibitory descending control and inhibitory interneurons, shifting the balance toward a hyperexcitable state. Hyperexcitability comprises increased sensitivity to noxious and innocuous stimuli, called hyperalgesia and allodynia, respectively, as observed in sickle mice.15-17,24,27,43-45
Electrophysiologic recordings in BERK sickle mice revealed increased excitability of spinal dorsal horn nociceptive neurons, demonstrated by enlarged receptive fields, exaggerated rate of spontaneous activity, increased responsiveness and prolonged after-discharges after mechanical stimulus, and lower mechanical threshold compared with BERK controls.44 We observed the activation of signaling pathways involved in neuronal hyperexcitability, including mitogen-activated protein kinases, c-Jun kinase, p42/p44 extracellular receptor kinase, and p38, as well as signal transducer and activator of transcription 3, in the spinal cords of sickle mice. We also found elevated reactive oxygen species (ROS), SP, and activated microglial and astrocytic cells with increased glial fibrillary acidic protein (GFAP) in the dorsal horn of the spinal cord of sickle mice.26 Interestingly, coenzyme Q10 (CoQ10) and curcumin with antioxidant and anti-inflammatory properties reduced spinal ROS, SP, and microglial and astroglial activation accompanied by reduced GFAP and attenuated hyperalgesia in BERK sickle mice. In patients with SCD, CoQ10 treatment decreased VOCs and circulating thiobarbituric acid reactive substances (indicative of oxidative stress).46 Higher circulating GFAP was reported in children with SCD compared with controls.25 Thus, heightened oxidative stress and neuroimmune interactions may contribute to neuronal hyperexcitability and chronic pain, pathways that may provide targets for novel therapeutic strategies.
This hyperexcitable state in the spinal cord may be a continuum of peripheral nociceptor sensitization in sickle mice, because enhanced excitability of primary afferents was observed in peripheral skin nerve preparations in response to mechanical stimuli, via activation of TRPV1 channels.24 Electrophysiologic recordings performed by us in tibial afferent nerve fibers in anesthetized BERK sickle mice showed elevated spontaneous activity as well as activity evoked by mechanical and thermal stimuli.45
In patients with SCD, quantitative sensory testing demonstrated heightened sensitivity to mechanical and thermal (heat and cold) stimuli, suggestive of central sensitization.19-23 Neuroimaging studies in patients with SCD showed higher pronociceptive connectivity and lower antinociceptive connectivity in the default mode network (DMN) of patients with longer pain-related hospitalization stays compared with those with shorter stays.47 Electroencelography recordings concurrently with functional magnetic resonance imaging performed by our group in patients with SCD elucidated the neurophysiologic manifestations of resting stage networks including the DMN.48 Higher activity was observed on electroencelography recordings in patients during rest in pain-processing regions, consistent with functional magnetic resonance imaging findings of reduced DMN activity and enhanced activity in pain-processing regions compared with control subjects.
In agreement with earlier findings, we found significantly reduced voxels in patients with SCD in the executive control network region involved in cognitive functions including perception and decision making.48 Poor cognitive function and alterations in the executive control network have been observed in patients with SCD.49-51 Simultaneously, psychosocial stress, cognitive impairment, sleep disorder, and pathobiological factors including inflammation, which exist in SCD, contribute to the maintenance and exaggeration of chronic pain.
Thus, observations in sickle mice and patients with SCD suggest peripheral nociceptor sensitization, central neuronal hyperexcitability and activation of signaling mechanisms, and alterations in brain function and activity suggestive of central sensitization. We speculate that central sensitization may contribute to the variability in pain intensity and medically refractory pain in SCD. The potential therapeutic relevance of these observations is that centrally mediated pain states are more responsive to serotonin-norepinephrine reuptake inhibitors and modulators of central mechanisms, whereas peripheral and nociceptive pain states may respond better to opioids and nonsteroidal analgesics.
Neurogenic inflammation
Activated C-fibers release vasoactive and proinflammatory neuropeptides, including SP and calcitonin gene–related peptide (CGRP), that stimulate arteriolar vasodilation and vascular leakage. SP and CGRP transported orthodromically to second-order neurons also contribute to central sensitization and lead to peripheral sensitization upon being released antidromically to peripheral nerve fibers (Figure 1). These vasoactive and proinflammatory neuropeptides may promote vascular dysfunction by increasing vascular permeability as well as nociceptor activation, thus contributing to multiple features of SCD including dactylitis and pain. Neurogenic inflammation also occurs in sickle mice, as discussed below.27,52 Because the ability of standard anti-inflammatory agents to ameliorate neurogenic inflammation and resulting pain is limited, novel agents that specifically target this pathway will likely need to be developed and tested in SCD.
Opioids in SCD
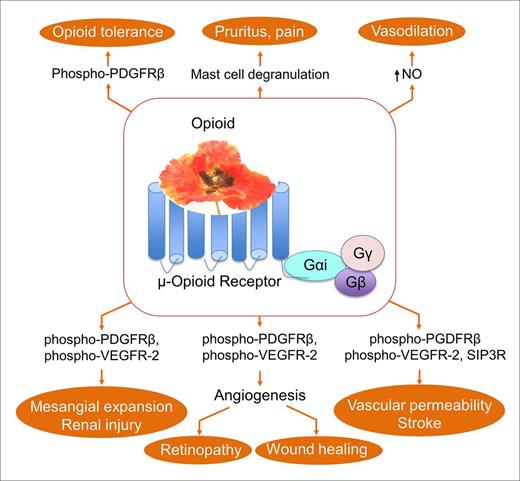
Opioids remain the common treatment of acute and chronic pain in SCD.1 Because opioids are required in comparatively larger doses and for longer duration compared with those used for analogous pain in other conditions, optimizing benefits and minimizing adverse effects are critical in SCD. Darbari et al demonstrated increased clearance of morphine in patients with SCD, which may contribute to the increased opioid requirement to treat pain in SCD.53 Mechanisms and nonanalgesic effects of opioids in SCD have been reviewed by us in detail in another review and are summarized here (Figure 2).18 Opioids adversely alter red blood cell (RBC) deformability, dehydration, rheology, and membrane structure. Chronic morphine treatment led to the exaggeration of renal pathology and impaired renal function in sickle mice.54 We have shown that morphine via the μ-opioid receptor (MOR) co-activates receptor tyrosine kinases (RTKs) for vascular endothelial growth factor receptor 2 (VEGFR-2) and platelet-derived growth factor β (PDGFR-β). Spinal PDGFR-β co-activation has been suggested to augment opioid tolerance in rats, which is ameliorated by the RTK inhibitor imatinib.55 In addition, the interaction of morphine with Toll-like receptor 4 in the CNS has been demonstrated to lead to opioid-induced hyperalgesia.56,57 These observations suggest that drugs targeting signaling pathways/RTKs, in combination with opioids, may offer the potential to improve opioid analgesia and reduce adverse effects.
μ-Opioid receptor–mediated mechanisms and functions of opioids relevant to sickle cell disease. Binding of opioids to μ-opioid receptors activates multiple signaling pathways that may exaggerate several complications, including increased vascular permeability, endothelial specific activity, stroke, vasodilation, opioid tolerance, retinopathy, renal pathology, and hyperalgesia. The center poppy represents analgesic opioids, including morphine, hydromorphone, and fentanyl. SIP3R, sphingosine 1 phosphate receptor 3.
μ-Opioid receptor–mediated mechanisms and functions of opioids relevant to sickle cell disease. Binding of opioids to μ-opioid receptors activates multiple signaling pathways that may exaggerate several complications, including increased vascular permeability, endothelial specific activity, stroke, vasodilation, opioid tolerance, retinopathy, renal pathology, and hyperalgesia. The center poppy represents analgesic opioids, including morphine, hydromorphone, and fentanyl. SIP3R, sphingosine 1 phosphate receptor 3.
Mechanism-based targets of sickle pain
Two approaches may be taken, individually or simultaneously, to treat pain in SCD. The first strategy is to use disease-modifying therapies that target the sickle pathobiology underlying pain. The second strategy is to develop analgesics that target the CNS. Because some crossover may occur between the 2 approaches, consideration of multimodal therapies is also required.
Possible mechanism-based targets of acute and chronic sickle pain are shown in Figure 1, and pharmacologic/alternative strategies are described in Table 3. Mechanisms of sickle pain are still emerging. Here, we describe some common mechanisms that have potential clinical relevance for developing improved analgesic strategies.
Pharmacologics/dietary supplements targeting pain mechanisms tested in mice and/or patients with sickle cell disease
| Generic name . | Mechanistic target . | Action . | Sickle subjects . | |
|---|---|---|---|---|
| Mice . | Humans . | |||
| A-425619 | TRPV1 | Antagonist | X | |
| AT-200 | NOP/R and MOR | Agonist | X | |
| Bosentan | ET-A and ET-B receptors | Antagonist | X | X |
| Coenzyme Q10 | Oxidative stress | Inhibitor | X | X |
| CP55940 | CB1R and CB2R | Agonist | X | |
| Crizanlizumab | P-selectin | Antibody | X | |
| Cromolyn | Mast cell | Stabilizer | X | X |
| Curcumin | ROS | Inhibitor | X | X |
| Duloxetine | Serotonin and norepinephrine reuptake transporter | Inhibitor | X | X |
| HC-030031 | TRPA1 | Antagonist | X | |
| Imatinib | Mast cell/c-kit/RTK | Inhibitor | X | X |
| KN-93 | CaMKII | Inhibitor | X | |
| l-arginine | NOS | Substrate | X | |
| l-glutamine | Oxidative stress | Antioxidant | X | |
| Lovastatin | HMG-CoA reductase | Inhibitor | X | |
| PEG-ADA | Adenosine | Inhibitor | X | |
| Rapamycin | mTOR | Inhibitor | X | |
| HbF | Inducer | |||
| Simvastatin | HMG-CoA reductase, calpain-1 | Inhibitor | X | X |
| Sivelestat | Neutrophil elastase | Inhibitor | X | |
| TAK-242 | TLR4 | Inhibitor | X | |
| Trifluoperazine | CaMKIIα | Inhibitor | X | X |
| ω-3 fatty acid | Oxidative stress | Antioxidant | X | X |
| Generic name . | Mechanistic target . | Action . | Sickle subjects . | |
|---|---|---|---|---|
| Mice . | Humans . | |||
| A-425619 | TRPV1 | Antagonist | X | |
| AT-200 | NOP/R and MOR | Agonist | X | |
| Bosentan | ET-A and ET-B receptors | Antagonist | X | X |
| Coenzyme Q10 | Oxidative stress | Inhibitor | X | X |
| CP55940 | CB1R and CB2R | Agonist | X | |
| Crizanlizumab | P-selectin | Antibody | X | |
| Cromolyn | Mast cell | Stabilizer | X | X |
| Curcumin | ROS | Inhibitor | X | X |
| Duloxetine | Serotonin and norepinephrine reuptake transporter | Inhibitor | X | X |
| HC-030031 | TRPA1 | Antagonist | X | |
| Imatinib | Mast cell/c-kit/RTK | Inhibitor | X | X |
| KN-93 | CaMKII | Inhibitor | X | |
| l-arginine | NOS | Substrate | X | |
| l-glutamine | Oxidative stress | Antioxidant | X | |
| Lovastatin | HMG-CoA reductase | Inhibitor | X | |
| PEG-ADA | Adenosine | Inhibitor | X | |
| Rapamycin | mTOR | Inhibitor | X | |
| HbF | Inducer | |||
| Simvastatin | HMG-CoA reductase, calpain-1 | Inhibitor | X | X |
| Sivelestat | Neutrophil elastase | Inhibitor | X | |
| TAK-242 | TLR4 | Inhibitor | X | |
| Trifluoperazine | CaMKIIα | Inhibitor | X | X |
| ω-3 fatty acid | Oxidative stress | Antioxidant | X | X |
ETA and ETB, endothelin A and endothelin B; CB1R/CB2R, cannabinoid receptor 1/2; HMG-CoA reductase, 3-hydroxy-3-methyl-glutaryl–coenzyme reductase; NE, norepinephrine; NOS, nitric oxide synthase; PEG-ADA, polyethylene glycol–modified adenosine deaminase; TRPA1, transient receptor potential cation channel subfamily A, member 1.
Disease-modifying targets
Leukocytes contribute to vaso-occlusion, leading to episodes of acute pain
P-selectin on endothelium and platelets facilitates the adhesion of RBCs and leukocytes, respectively, in the process of vaso-occlusion. Treatment of patients with SCD with the P-selectin–targeted humanized monoclonal antibody crizanlizumab (SelG1) led to a significant reduction in pain crises compared with placebo in a 12-month phase 2, double-blind, randomized, placebo-controlled clinical trial.58
Leukocytes may also contribute to chronic pain. Elastase released upon neutrophil activation and nuclear extracellular trap formation leads to inflammation and hyperalgesia via activation of protease-activated receptor 2 (PAR2) and TRPV4 on peripheral nerve endings in mice.59 Nuclear extracellular traps have been demonstrated in BERK sickle mice as well as in patients with SCD.60 We found that SerpinA3N, a regulator of elastase activity, is downregulated and elastase activity is increased in the DRG of sickle mice compared with controls.61 Elevated neutrophil elastase has been reported in the plasma of patients with SCD compared with healthy controls, with a significant increase during a VOC.62,63 Treatment of BERK sickle mice with the elastase inhibitor sivelestat ameliorated spontaneous/tonic (chronic) hyperalgesia.61 Sivelestat treatment also ameliorated neuropathic pain in a rodent model of diabetes with enhanced activity of lymphocyte-derived elastase in the DRG.64
Furthermore, increased endothelin-1 (ET-1) gene expression in the DRG has been observed in BERK sickle mice.65 Inhibition of neutrophil migration and recruitment by an ET-1 receptor antagonist reduced pain hypersensitivity in Townes sickle mice (A. Kutlar, Medical College of Georgia, personal communication, 2 May 2017). It is thus conceivable that the ET1R/ET-1 axis exerts a direct effect on nociceptor activity and may act via neutrophil recruitment and activation, thus contributing to hyperalgesia directly and through a disease-modifying mechanism, respectively.
Together, these findings indicate that the activation of leukocytes in SCD may directly or indirectly contribute to chronic as well as acute pain and that pharmacologic agents targeting neutrophils may help ameliorate pain.
Mast cells as targets to treat neurogenic inflammation and pain
Our findings suggest that mast cell degranulation releases tryptase, which stimulates PAR2 and TRPV1 channels on peripheral nerve endings, leading to the release of SP from nerve terminals in BERK sickle mice.27 Consequently, SP via neurokinin 1 receptors stimulates arteriolar dilatation and vascular permeability, leading to neurogenic inflammation and pain. Released SP also activates mast cells, leading to a vicious cycle of mast cell activation, neurogenic inflammation, and pain. Patients with SCD with chronic pain show significantly increased circulating tryptase, a marker of mast cell degranulation, compared with normal controls and patients with SCD without pain.31
In BERK sickle mice, the inhibition of mast cell degranulation with imatinib or deletion of mast cells led to a significant amelioration of chronic and H/R-evoked hyperalgesia.27 Cromolyn, a mast cell stabilizer, and imatinib, a c-kit inhibitor, reduced neurogenic inflammation and cytokine release in the skin. Notably, cromolyn did not reduce hyperalgesia, but enhanced the analgesic effect of a suboptimal dose of morphine in attenuating chronic pain. Because morphine stimulated the release of SP from both the skin and DRG of sickle mice, it is likely that although morphine imparts analgesia via centrally mediated mechanisms, it concomitantly promotes hyperalgesia by activating mast cells.
In patients with SCD, cromolyn significantly reduced RBC sickling in vitro after H/R compared with placebo, but cromolyn sodium nasal spray did not significantly relieve pain.66 Intriguingly, imatinib reduced the incidence of VOCs in a patient with acute myeloid leukemia and SCD, without having an effect on hematologic variables or nitric oxide (NO) or hemoglobin F (HbF), suggesting that mechanisms besides these are involved.67 In sickle mice, imatinib significantly reduced the inflammatory cytokines IL-6, MCP-1, TNF-α, GMCSF, and RANTES.27 Elevated adenosine in sickle mice led to increased IL-6–mediated hypersensitivity via ADORA2B signaling in myeloid cells, further implicating mast cells in neuronal hypersensitivity.68 These findings provide the rationale for examining the role of mast cells in patients with SCD and for evaluating the efficacy of imatinib in ameliorating VOCs and acute and chronic pain.
Factors influencing mast cell activity influence pain
Deletion of the calpain-1 protease inhibits mast cell activation in wild-type mice.69 In Townes sickle mice, deletion of calpain-1 attenuated chronic hyperalgesia but not that evoked by H/R.70 Independently, the role of calpain-1 merits further investigation in chronic pain because of its catalytic activity in proteolyzing Ca2+/calmodulin-dependent protein kinase II (CaMKII) in neurons to an active core fragment, which plays a critical role in neurotransmission.71 CaMKIIα is significantly activated in both the spinal cord and DRG of BERK sickle mice, contributing to the induction and maintenance of hyperalgesia.72 The administration of the CaMKII inhibitor KN-93 reduced responses to mechanical and thermal stimuli in BERK sickle mice.72 CaMKIIα may be involved in progression from acute to chronic pain by inducing persistent changes in peripheral nerve terminals, which prolong the hyperalgesic effects of proinflammatory markers.73 Indeed, patients with SCD given a single dose of trifluoperazine, a CaMKIIα inhibitor, reported a noticeable analgesic effect.74 Calpain-1 protein expression and calpain activity in mice can be decreased by statins such as simvastatin.75 Simvastatin also inhibited neutrophil activation induced by antineutrophil cytoplasmic antibodies.76 In patients with SCD, simvastatin treatment led to decreased frequency of pain, reduced analgesic use, and decreased high-sensitivity C-reactive protein, E-selectin, VEGF, and soluble intercellular adhesion molecules 1 and 3 but had no effect on the intensity of reported pain.77 Combining simvastatin with hydroxyurea increased the effects on molecular markers and pain. Thus, statins may reduce the activity of neutrophils as well as mast cells.
Targeting mast cells directly and/or through other regulatory mechanisms provides a clinically testable strategy because of the availability of Food and Drug Administration–approved drugs including cromolyn, imatinib, and statins.
Other disease-modifying strategies
Strategies to prevent VOCs have been recently reviewed, which include antioxidants l-glutamine (Endari; Emmaus Medical, Torrance, CA), and ω-3 fatty acids as promising therapeutic agents to prevent VOCs.78
Targeting analgesic mechanisms
Cannabinoid-based mechanisms to improve analgesia
Cannabinoids are well known for their activity in the CNS and their direct antinociceptive effects.79 CP55940, a nonselective cannabinoid receptor agonist significantly reduced cutaneous mast cell degranulation, neurogenic inflammation, and circulating levels of SP under normoxia and after H/R in BERK sickle mice.52 Circulating inflammatory marker serum amyloid protein and mast cell activation markers tryptase and β-hexosaminidase were significantly reduced in CP55940-treated sickle mice compared with vehicle. Mast cell degranulation and neurogenic inflammation under H/R were inhibited by CB1R as well as CB2R agonists. However, CB2R agonists were not able to attenuate chronic or H/R-evoked acute hyperalgesia in BERK sickle mice. The deletion of CB2R from BERK sickle mice did not diminish the analgesic effect of CP55940, suggesting that cannabinoid analgesia in sickle mice requires activation of CB1R. Therefore, cannabis-based medications with CB1R activity may be beneficial for chronic and acute sickle pain through a disease-modifying mechanism and through a neurally mediated analgesic mechanism. A double-blind, placebo-controlled crossover trial is ongoing to determine the effect of vaporized cannabis on pain and circulating inflammatory and nociceptive markers in patients with SCD (clinicaltrials.gov, NCT01771731).
Another potential mechanism targeting nociception is the nociceptin receptor (NOP/R), a member of the opioid receptor family. A small-molecule NOP/R agonist AT-200 with partial affinity to MOR ameliorated chronic and acute hyperalgesia without causing tolerance in sickle mice.80 NOP/R agonists are in clinical trial for chronic pain.81 Because AT-200 inhibited mast cell degranulation and neurogenic inflammation, it may also influence sickle pathobiology.
Strategies to increase NO
Reduced NO bioavailability has been suggested to contribute to sickle pathobiology.82 Arginine is a substrate for NO synthase, leading to the generation of NO. During a VOC, plasma arginine concentration decreases significantly in association with decreased NO metabolites in patients with SCD.83 A single dose of arginine supplementation led to an increase in NO during VOCs but not at steady state in patients with SCD.84 l-arginine treatment led to reduced pain intensity and had an opioid-sparing effect without causing any adverse effects in patients admitted with a VOC.85 The function of l-arginine bioavailability was also suggested to be independent of NO levels in these studies. Hydroxyurea was shown to elicit its anti-inflammatory effects via NO-mediated mechanisms.86 However, hydroxyurea does not itself have an analgesic effect. Both pro- and antinociceptive activity of NO has been suggested in the CNS.87 In a multicenter, placebo-controlled, double-blind trial of inhaled NO over 72 hours in patients with SCD presenting with a VOC, NO had no effect on acute pain or parenteral opioid requirement.88 Inhaled NO did not increase adverse events in this trial. On the other hand, a multicenter clinical trial that used sildenafil intended to increase NO in subjects with SCD with elevated tricuspid jet velocities, a marker of pulmonary hypertension, led to increased hospital admissions due to pain and was stopped prematurely.89 Because sildenafil is a phosphodiesterase-5 inhibitor, it may have off-target effects, perhaps on central mechanisms of nociception. These observations indicate that careful selection of strategies targeting the SCD process versus those that target nociceptive pathways will be essential to prevent adverse effects of one on the other.
Newer strategies to increase HbF
Increased HbF is associated with reduced frequency of painful VOCs in SCD.47 Several novel genetic and pharmacologic strategies that increase HbF have been developed, including histone modification, DNA methylation, and BCL11a modification, but whether they directly reduce pain needs to be tested.90,91 HbF production was stimulated in Townes sickle mouse and human erythroid cells by rapamycin, an inhibitor of mechanistic target of rapamycin complex 1 (mTORC1). This was associated with reduced nociception in the sickle mice.92 Other mTOR inhibitors reduce ROS accumulation, increase RBC life span, reduce middle cerebral artery occlusion, reduce spleen size, and decrease iron accumulation in the liver and kidney of sickle mice.93,94 mTOR inhibition may therefore have multiple beneficial effects on SCD pathobiology in addition to stimulating HbF expression. It is possible that these effects may prevent or reduce pain evoked by the sickle microenvironment.
Nonpharmacologic interventions
Complementary and integrative medicine including acupuncture, dietary supplements, and mind-body systems are being increasingly used for treating chronic pain partly to reduce complications of long-term opioid use.95 We found that electro-acupuncture without anesthesia in awake BERK sickle mice led to a variable analgesic response, categorized into high, moderate, and low responses.96 These effects were correlative to a reduction in inflammatory cytokines, SP, and neurogenic inflammation in the periphery and signaling pathways of nociception in the spinal cord, thus influencing the sickle microenvironment as well as nociception centrally. Notably, a suboptimal dose of morphine coadministered with electro-acupuncture to moderate responders achieved analgesia equivalent to that in high responders. Another strategy described earlier using the dietary supplement curcumin given orally attenuated hyperalgesia significantly in BERK sickle mice.26
Although pain is a subjective conscious experience driven by somatosensory nociceptive activity, it can be modulated by cognitive and emotional modulators through higher brain centers involving the “pain neuromatrix” and neurochemical activity via the limbic descending/top-down mechanisms. In turn, pain may influence vascular physiology in SCD. Thermal pain impulses evoked peripheral microvascular constriction in patients with SCD.97,98 A significant reduction in microvascular flow occurred in anticipation of pain as soon as the subjects learned about the pain they were about to feel. It is conceivable that emotional stress involving the autonomic nervous system may contribute to VOCs. Therefore, targeting cognitive and neuromodulatory mechanisms may have a positive impact on vascular and neural physiology in SCD.
Future perspectives
The knowledge gained thus far has provided the directions for future efforts to improve pain treatment in SCD. It is critical to understand how these mechanisms interact with each other and the relative contribution of each to sickle pain. Several mechanism-based agents identified thus far warrant testing in clinical trials. On the basis of the side effects and signaling pathways activated by opioids, cotreatment strategies of opioids with pharmacologic adjuvants and/or complementary and integrative medicine approaches are required to reduce opioid dose and side effects. Strategies need to be developed to incorporate these novel strategies with standard treatment of SCD, such as hydroxyurea.
Acknowledgments
We thank Thomas Coates, Raj Badgaiyan, Lonnie Zeltzer, Samir Ballas, Abdullah Kutlar, Deepika Darbari, William R. Smith, and Pankaj Gupta for fruitful discussions and/or critical review of the manuscript and Michael Franklin for editorial suggestions. We apologize for our inability to include many of the studies due to space limitations, but we deeply appreciate their contribution to the study of pain/SCD.
This work was supported by National Institutes of Health grant UO1 HL117664 (to K.G.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: K.G. conceived, designed, wrote, and edited the review; H.T. performed literature search, cowrote the review, and prepared the graphics; and M.G. cowrote the review.
Conflict-of-interest disclosure: H.T. declares no competing financial interest. M.G. declares no competing financial interest. K.G. has consulted for Fera Pharmaceuticals LLC and Tau tona Group. Off-label drug use: None disclosed.
Correspondence: Kalpna Gupta, Vascular Biology Center, Medicine–Hematology, Oncology, and Transplantation, University of Minnesota, Mayo Mail Code 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: gupta014@umn.edu.
References
Author notes
This article was selected by the Blood and Hematology 2017 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2017. It is reprinted in Hematology Am Soc Hematol Educ Program. 2017;2017:546-555.