Key Points
Venetoclax monotherapy at a daily dose up to 1200 mg has an acceptable safety profile in patients with relapsed/refractory MM.
Venetoclax monotherapy has demonstrated antimyeloma activity in patients with relapsed/refractory MM positive for t(11;14).
Abstract
Venetoclax is a selective, orally bioavailable BCL-2 inhibitor that induces cell death in multiple myeloma (MM) cells, particularly in those harboring t(11;14), which express high levels of BCL-2 relative to BCL-XL and MCL-1. In this phase 1 study, patients with relapsed/refractory MM received venetoclax monotherapy. After a 2-week lead-in with weekly dose escalation, daily venetoclax was given at 300, 600, 900, or 1200 mg in dose-escalation cohorts and 1200 mg in the safety expansion. Dexamethasone could be added on progression during treatment. Sixty-six patients were enrolled (30, dose-escalation cohorts; 36, safety expansion). Patients received a median of 5 prior therapies (range, 1-15); 61% were bortezomib and lenalidomide double refractory, and 46% had t(11;14). Venetoclax was generally well tolerated. Most common adverse events included mild gastrointestinal symptoms (nausea [47%], diarrhea [36%], vomiting [21%]). Cytopenias were the most common grade 3/4 events, with thrombocytopenia (32%), neutropenia (27%), anemia (23%), and leukopenia (23%) reported. The overall response rate (ORR) was 21% (14/66), and 15% achieved very good partial response or better (≥VGPR). Most responses (12/14 [86%]) were reported in patients with t(11;14). In this group, ORR was 40%, with 27% of patients achieving ≥VGPR. Biomarker analysis confirmed that response to venetoclax correlated with higher BCL2:BCL2L1 and BCL2:MCL1 mRNA expression ratios. Venetoclax monotherapy at a daily dose up to 1200 mg has an acceptable safety profile and evidence of single-agent antimyeloma activity in patients with relapsed/refractory MM, predominantly in patients with t(11;14) abnormality and those with a favorable BCL2 family profile. Registered at www.clinicaltrials.gov: #NCT01794520.
Introduction
Despite advances in new and effective treatment strategies, multiple myeloma (MM) remains incurable, with inevitable relapse in the majority of patients. Development of novel agents with a unique mechanism of action that are active in relapsed/refractory MM will expand options for patients.1
The intrinsic apoptosis pathway is regulated by a balance between antiapoptotic (eg, BCL-2, BCL-XL, BCL-W, myeloid cell leukemia sequence [MCL]-1) and proapoptotic (eg, BAX, BAK, BIM, BID, NOXA) proteins.2-4 Normally, proapoptotic proteins are sequestered by BCL-2, BCL-XL, and/or MCL-1 and are prevented from inducing cell death.2 However, various toxic stimuli can result in their release and translocation to the mitochondrial outer membrane, leading to an increase in mitochondrial permeability and a cascade of signaling leading to cellular apoptosis.2 In myeloma cells, overexpression of antiapoptotic proteins is heterogeneous. BCL-2 has been shown to be overexpressed in a subset of myeloma cells and implicated in MM cell survival.5 BH3 profiling analysis confirmed a BCL-2 survival dependency for a subgroup of MM cells.6 Antagonizing BCL-2 function to induce apoptosis is therefore a compelling therapeutic approach in MM.
Venetoclax is a potent, selective, orally bioavailable inhibitor of BCL-2. Selective BCL-2 targeting with venetoclax has shown promising antitumor activity in a number of hematologic malignancies, including chronic lymphocytic leukemia, acute myeloid leukemia, and non-Hodgkin lymphoma.7-10 In vitro data showed a high sensitivity to venetoclax in human myeloma cell lines and primary MM samples that were positive for the (11;14) translocation, which correlated with higher ratios of BCL2 to MCL1 mRNA.11 t(11;14) is seen in 15% to 20% of patients with MM12,13 and is currently classified as a standard-risk genetic marker for newly diagnosed patients. Some recent studies have suggested that t(11;14) may be an intermediate-risk marker in MM.14,15 Interestingly, t(11;14) is also found in almost 40% of patients with primary plasma cell leukemia, an aggressive variant of MM.16
Here we report the safety, efficacy, pharmacokinetics, and exploratory biomarker results from an ongoing, open-label, phase 1 study of venetoclax monotherapy in patients with relapsed/refractory MM.
Methods
Study design
The open-label, dose-escalation, phase 1 M13-367 study (www.clinicaltrials.gov, NCT01794520) recruited patients with relapsed/refractory MM starting in October 2012 and is ongoing. Recruitment has been completed for the venetoclax monotherapy dose escalation and safety expansion cohort, and follow-up is ongoing. Data cutoff for this report is August 19, 2016. Primary objectives of the study were to evaluate the safety profile and pharmacokinetics and to determine the maximum tolerated dose and recommended phase 2 dose of venetoclax in relapsed/refractory MM. Secondary objectives included overall response rate (ORR), time to progression (TTP), duration of response (DOR), and exploratory biomarkers.
Patients
Patients with relapsed/refractory MM were enrolled (complete eligibility criteria are listed in supplemental Data, available on the Blood Web site). In brief, patients should have received at least 1 prior line of therapy to be included in the dose-escalation cohorts; for the safety expansion, patients must also have had prior treatment with both a proteasome inhibitor and an immunomodulatory drug. Patients must have had measurable disease at baseline, including monoclonal protein at least 1 g/dL in serum or at least 200 mg/24 hours in urine or serum immunoglobulin free light chain at least 10 mg/dL; adequate bone marrow, renal, and hepatic function; and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients were excluded if they had an active infection, history of significant medical illness within 6 months of study entry, or history of other active malignancies within 3 years of study entry.
Treatment
Venetoclax was administered orally, once daily. To mitigate risk for tumor lysis syndrome (TLS), a 2-week lead-in period was employed and TLS prophylaxis initiated for all patients, starting at least 72 hours before the first dose and at each dose increase (“Management of TLS”; supplemental Table 1). After the 2-week lead-in, in which patients received starting doses of venetoclax between 50 and 400 mg for the first week escalated to the target dose by week 3 (supplemental Figure 1), patients were treated on 21-day cycles with daily venetoclax given at final doses of 300, 600, 900, or 1200 mg in dose-escalation cohorts (3+3 design) and 1200 mg in the safety expansion (supplemental Figure 1). Patients who progressed during treatment could elect to add dexamethasone to venetoclax and continue on study.
Study assessments
Safety.
Assessments were conducted at screening and throughout the study and included adverse event (AE) monitoring, measurement of vital signs, physical examination, 12-lead electrocardiography, multiple-gated acquisition/2-dimensional echocardiogram, and clinical laboratory tests (“Additional safety methods”; supplemental Data). Dose-limiting toxicities were determined during the lead-in period plus the first cycle (21 days) of venetoclax treatment at the designated cohort dose. AEs occurring after cycle 1 were also evaluated by the investigator and the study medical monitor to determine whether they were dose limiting. Dose-limiting toxicities were defined as the following venetoclax-related AEs: grade 4 neutropenia lasting more than 7 days, grade 3 or 4 febrile neutropenia, grade 4 thrombocytopenia, grade 2 or higher bleeding associated with grade 3 or higher thrombocytopenia, unexpected grade 2 or higher toxicity requiring dose modification or delay of 1 week or more (eg, peripheral neuropathy), clinical TLS, and laboratory TLS if the metabolic abnormalities are considered clinically significant by the investigator; all other grade 3 or higher AEs were considered a dose-limiting toxicity with the exception of grade 3 thrombocytopenia without bleeding; grades 3 to 4 lymphopenia and leukopenia; grade 3 neutropenia; grade 3 nausea, vomiting, and/or diarrhea that is responsive to treatment; grade 3 or 4 hyperuricemia or hypocalcemia; or grade 3 hyperkalemia if transient (lasting <48 hours) and without manifestations of clinical TLS.
Efficacy.
Clinical responses as well as clinical disease progression or relapse were assessed by the investigator, using the International Myeloma Working Group criteria (supplemental Table 2).17,18 The efficacy assessments reported here for patients who added dexamethasone to venetoclax on progression were limited to the period with venetoclax monotherapy before progression, unless otherwise specified.
Pharmacokinetics and exploratory biomarkers.
Pharmacokinetic assessments and results, as well as exploratory biomarker methods, are described in the supplemental Data (supplemental Methods; supplemental Figure 2; supplemental Table 4).
Study oversight
The study was designed jointly by the investigators and sponsor according to Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable regulations, with institutional review board approval at all study sites. The sponsor conducted data analyses and investigators had full access to data for review. The first draft of the manuscript was written by a medical writer employed by AbbVie, with author input. Subsequent drafts were prepared by all authors and a medical writer. The authors made the decision to submit the manuscript for publication and attest to adherence to the study protocol and accuracy of data reported. All patients provided written informed consent.
Statistical analyses
Patients who received at least 1 dose of venetoclax were included in all analyses. Efficacy was also evaluated by t(11;14) translocation status. SAS software (SAS Institute, Inc., Cary, NC) was used for all statistical analyses.
Results
Patient demographics and clinical characteristics
Sixty-six patients were enrolled, with 30 in dose-escalation cohorts and 36 in the safety expansion. The median age was 63 years (range, 31-79 years), and patients received a median of 5 prior therapies (range, 1-15 prior therapies). The majority of patients (61%) were refractory to both bortezomib and lenalidomide. Thirty (46%) patients were positive for t(11;14), of which 5 also had chromosome 17p deletion, 11 had chromosome 13q deletion, and 8 were identified as hyperdiploid. Although the study did not select for t(11;14) MM, a high proportion of patients with this abnormality were enrolled by investigators based on previously available data showing activity of venetoclax in t(11;14)-positive myeloma cells.11 Baseline characteristics, including by t(11;14) status, are provided in Table 1.
Patient demographics and clinical characteristics
| . | All patients (N = 66) . | t(11;14) (n = 30) . | Non-t(11;14) (n = 36) . |
|---|---|---|---|
| Age, median (range), years | 63 (31-79) | 63 (31-77) | 64 (41-79) |
| Male, n (%) | 30 (46) | 18 (60) | 12 (33) |
| White, n/N (%) | 59/63 (94) | 28/29 (97) | 31/34 (91) |
| Time from MM diagnosis to study entry, median (range), months | 71 (11-185) | 50 (12-178) | 79 (11-185) |
| ISS stage, n/N (%) | |||
| Stage I | 24/63 (38) | 10/27 (37) | 14 (39) |
| Stage II or III | 39/63 (62) | 17/27 (63) | 22 (61) |
| Cytogenetic abnormalities, n (%) | |||
| t(4;14) translocation | 6 (9) | 0 | 6 (17) |
| 17p deletion | 12 (18) | 5 (17) | 7 (19) |
| 13q deletion | 32 (48) | 11 (37) | 21 (58) |
| Hyperdiploid | 27 (41) | 8 (27) | 19 (53) |
| Prior therapies,*n (%) | |||
| No. of prior therapies, median (range) | 5 (1-15) | 5 (1-10) | 5 (1-15) |
| Autologous stem cell transplant | 50 (76) | 21 (70) | 29 (81) |
| Bortezomib | 62 (94) | 28 (93) | 34 (94) |
| Refractory | 46 (70) | 22 (73) | 24 (67) |
| Lenalidomide | 62 (94) | 27 (90) | 35 (97) |
| Refractory | 51 (77) | 23 (77) | 28 (78) |
| Bortezomib/lenalidomide refractory | 40 (61) | 20 (67) | 20 (56) |
| Carfilzomib | 25 (38) | 13 (43) | 12 (33) |
| Refractory | 20 (30) | 11 (37) | 9 (25) |
| Pomalidomide | 39 (59) | 21 (70) | 18 (50) |
| Refractory | 35 (53) | 19 (63) | 16 (44) |
| Refractory to last prior therapy | 52 (79) | 26 (87) | 26 (72) |
| . | All patients (N = 66) . | t(11;14) (n = 30) . | Non-t(11;14) (n = 36) . |
|---|---|---|---|
| Age, median (range), years | 63 (31-79) | 63 (31-77) | 64 (41-79) |
| Male, n (%) | 30 (46) | 18 (60) | 12 (33) |
| White, n/N (%) | 59/63 (94) | 28/29 (97) | 31/34 (91) |
| Time from MM diagnosis to study entry, median (range), months | 71 (11-185) | 50 (12-178) | 79 (11-185) |
| ISS stage, n/N (%) | |||
| Stage I | 24/63 (38) | 10/27 (37) | 14 (39) |
| Stage II or III | 39/63 (62) | 17/27 (63) | 22 (61) |
| Cytogenetic abnormalities, n (%) | |||
| t(4;14) translocation | 6 (9) | 0 | 6 (17) |
| 17p deletion | 12 (18) | 5 (17) | 7 (19) |
| 13q deletion | 32 (48) | 11 (37) | 21 (58) |
| Hyperdiploid | 27 (41) | 8 (27) | 19 (53) |
| Prior therapies,*n (%) | |||
| No. of prior therapies, median (range) | 5 (1-15) | 5 (1-10) | 5 (1-15) |
| Autologous stem cell transplant | 50 (76) | 21 (70) | 29 (81) |
| Bortezomib | 62 (94) | 28 (93) | 34 (94) |
| Refractory | 46 (70) | 22 (73) | 24 (67) |
| Lenalidomide | 62 (94) | 27 (90) | 35 (97) |
| Refractory | 51 (77) | 23 (77) | 28 (78) |
| Bortezomib/lenalidomide refractory | 40 (61) | 20 (67) | 20 (56) |
| Carfilzomib | 25 (38) | 13 (43) | 12 (33) |
| Refractory | 20 (30) | 11 (37) | 9 (25) |
| Pomalidomide | 39 (59) | 21 (70) | 18 (50) |
| Refractory | 35 (53) | 19 (63) | 16 (44) |
| Refractory to last prior therapy | 52 (79) | 26 (87) | 26 (72) |
ISS, International Staging System.
Percentages for refractory status based on total study population.
Disposition
Median time on study for all patients was 3.3 months (range, 0.2-27 months), with median time on venetoclax monotherapy of 2.5 months (range, 0.2-25 months). In the t(11;14) group, median time on study was 7.8 months (range, 0.4-25 months), which was the same as time receiving venetoclax monotherapy. For 17 patients [9 with t(11;14)], dexamethasone was added after disease progression, and median time receiving venetoclax plus dexamethasone was 1.4 months (range, 0.1-13 months). Fifty-five (83%) patients discontinued the study, with 42 for disease progression and 5 for AEs; in addition, 2 withdrew consent, 1 was lost to follow-up, and 5 discontinued for reasons not specified. Eight patients with t(11;14) and 3 in the non-t(11;14) group are currently still on study (Figure 1). Three patients required dose reductions because of AEs, and 20 required temporary interruption of venetoclax dosing because of AEs. Eight deaths were reported, with 6 resulting from disease progression, 1 resulting from lung disorder, and 1 resulting from brain hemorrhage after trauma. Supplemental Table 4 provides details on reasons for discontinuations and dose adjustments, and supplemental Table 5 shows data by target dose.
Time on study by t(11;14) status and response to treatment. Patients in the non-t(11;14) group were identified either as not having t(11;14) or having undetermined cytogenetics. Venetoclax monotherapy is shown in red for patients with t(11;14) and in blue for patients in the non-t(11;14) group. Seventeen patients who progressed during monotherapy elected to receive venetoclax plus dexamethasone (shown in green) and stayed on study. Clinical responses are also shown on the left axis. *Patient discontinued with no response data. #Patient discontinuation was related to disease progression. CR, complete response; MR, minimal response; PD, progressive disease; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
Time on study by t(11;14) status and response to treatment. Patients in the non-t(11;14) group were identified either as not having t(11;14) or having undetermined cytogenetics. Venetoclax monotherapy is shown in red for patients with t(11;14) and in blue for patients in the non-t(11;14) group. Seventeen patients who progressed during monotherapy elected to receive venetoclax plus dexamethasone (shown in green) and stayed on study. Clinical responses are also shown on the left axis. *Patient discontinued with no response data. #Patient discontinuation was related to disease progression. CR, complete response; MR, minimal response; PD, progressive disease; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
Pharmacokinetics
Peak venetoclax concentrations were attained at 2 to 8 hours postdose (supplemental Figure 2). Venetoclax half-life could not be estimated in the study because of the limited sampling after the time to maximum observed plasma concentration (Tmax). Venetoclax mean maximum observed plasma concentration (Cmax) and area under the plasma concentration-time curve from time 0 to 24-hour dose interval (AUC24) values ranged from 0.912 to 3.74 μg/mL and 13.4 to 59.0 μg⋅hr/mL, respectively (supplemental Table 8).
Safety profile
All patients experienced at least 1 AE, with the most common being mild to moderate gastrointestinal toxicities (nausea [47%], diarrhea [36%], and vomiting [21%]). Hematologic toxicities were the most common grade 3 or 4 AEs (thrombocytopenia [26%], neutropenia [21%], anemia [14%], and leukopenia [14%]; Table 2). Serious AEs in 2% or more of patients included pneumonia (8%), sepsis (5%), cough, hypotension, pain, and pyrexia (3% each). Two patients experienced dose-limiting toxicities at the 600-mg dose. One patient had grade 3 nausea and grade 2 abdominal pain after 1 week at 600-mg daily dosing; both AEs resolved with discontinuation of venetoclax. The second patient had grade 3 vomiting and epigastralgia reported on the first day of 600-mg dosing that were considered dose limiting. This patient had hepatomegaly and cholestasis at the time of the event. The liver biopsy was inconclusive of relation to study drug. This event occurred in a context of disease progression. A decision was made to continue the study and escalate cautiously to the 900-mg target dose cohort, with a lead-in week at 600 mg and no further expansion of the 600-mg cohort. The maximum tolerated dose was not reached, and the safety expansion proceeded with a dose of 1200 mg daily, the planned maximum dose. No major differences in safety events were observed across target doses (supplemental Table 5), and no events of TLS were reported.
Summary of adverse events
| Event, n (%) . | Any grade (N = 66)* . | Grade 3/4 (N = 66)* . |
|---|---|---|
| Any adverse event | 66 (100) | 45 (68) |
| Nonhematologic adverse events | ||
| Nausea | 31 (47) | 2 (3) |
| Diarrhea | 24 (36) | 2 (3) |
| Fatigue | 18 (27) | 3 (5) |
| Back pain | 14 (21) | 5 (8) |
| Vomiting | 14 (21) | 2 (3) |
| Hematologic adverse events | ||
| Thrombocytopenia | 21 (32) | 17 (26) |
| Neutropenia | 18 (27) | 14 (21) |
| Anemia | 15 (23) | 9 (14) |
| Leukopenia | 15 (23) | 9 (14) |
| Lymphopenia | 12 (18) | 10 (15) |
| Serious adverse event† | Total | |
| Any serious adverse event | 21 (32) | |
| Pneumonia | 5 (8) | |
| Sepsis | 3 (5) | |
| Cough | 2 (3) | |
| Hypotension | 2 (3) | |
| Pain | 2 (3) | |
| Pyrexia | 2 (3) | |
| Event, n (%) . | Any grade (N = 66)* . | Grade 3/4 (N = 66)* . |
|---|---|---|
| Any adverse event | 66 (100) | 45 (68) |
| Nonhematologic adverse events | ||
| Nausea | 31 (47) | 2 (3) |
| Diarrhea | 24 (36) | 2 (3) |
| Fatigue | 18 (27) | 3 (5) |
| Back pain | 14 (21) | 5 (8) |
| Vomiting | 14 (21) | 2 (3) |
| Hematologic adverse events | ||
| Thrombocytopenia | 21 (32) | 17 (26) |
| Neutropenia | 18 (27) | 14 (21) |
| Anemia | 15 (23) | 9 (14) |
| Leukopenia | 15 (23) | 9 (14) |
| Lymphopenia | 12 (18) | 10 (15) |
| Serious adverse event† | Total | |
| Any serious adverse event | 21 (32) | |
| Pneumonia | 5 (8) | |
| Sepsis | 3 (5) | |
| Cough | 2 (3) | |
| Hypotension | 2 (3) | |
| Pain | 2 (3) | |
| Pyrexia | 2 (3) | |
Reported events of neutropenia include the preferred terms of neutropenia and decreased neutrophil counts, thrombocytopenia includes the preferred terms of thrombocytopenia and decreased platelet counts, and leukopenia includes leukopenia and decreased white blood cells.
Adverse events for 20% or more of patients for any grade event or for 10% or more with grade 3/4 adverse events are listed.
Serious adverse events in 2% or more of patients are listed.
Efficacy
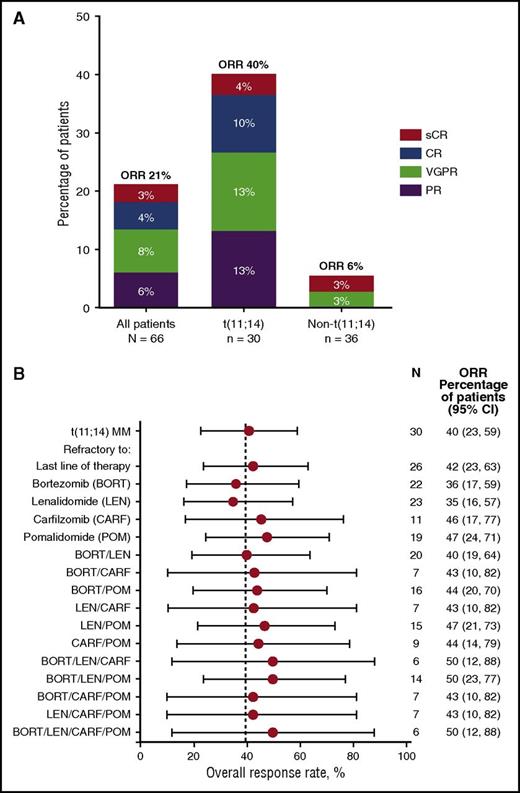
Among all patients, 14/66 (21%) achieved an overall response (partial response [PR] or better) on venetoclax monotherapy, with 10 (15%) achieving very good PR or better (≥VGPR) (Figure 2A; supplemental Table 6). Of these 14 responders, 12 had t(11;14) MM. The ORR and rate of ≥VGPR among the 30 patients in the t(11;14) group were 40% and 27%, respectively (1 stringent complete response, 3 complete response, 4 VGPR). Median DOR and median TTP for all patients were 9.7 months (95% CI, 7.0, not reached) and 2.6 months (95% CI, 1.9-4.7), respectively. Similar to the total study population, the median DOR for patients with t(11;14) was 9.7 months (95% CI, 6.3, not reached). However, in patients with t(11;14), the median TTP was 6.6 months (95% CI, 3.9-10.2 months) compared with a median TTP of 1.9 months (95% CI, 1.2-2.3 months) in the non-t(11;14) group (Figure 3). The median TTP in patients with t(11;14) who achieved PR and ≥VGPR response was 8.6 and 11.5 months, respectively (supplemental Figure 3). The 2 clinical responses in the non-t(11;14)/undetermined cytogenetics group were reported in 1 patient with translocation of chromosome 14 with an unidentified partner (stringent complete response, DOR 9.5 months) and in a second patient with missing cytogenetics data (VGPR, DOR, 7.2 months; Figure 3).
ORR by t(11;14) status. (A) Response data for all patients and by t(11;14) translocation status. Patients in the non-t(11;14) group were identified as not having t(11;14) or undetermined cytogenetics. ORR indicates a response of PR or better. (B) Response rates in patient subgroups based on refractoriness to prior therapies among 30 patients with t(11;14) MM. 95% CIs are provided. No patients enrolled in the study had prior exposure to daratumumab. Dotted line indicates an ORR of 40% achieved in patients with t(11;14) MM on this study. CI, confidence interval.
ORR by t(11;14) status. (A) Response data for all patients and by t(11;14) translocation status. Patients in the non-t(11;14) group were identified as not having t(11;14) or undetermined cytogenetics. ORR indicates a response of PR or better. (B) Response rates in patient subgroups based on refractoriness to prior therapies among 30 patients with t(11;14) MM. 95% CIs are provided. No patients enrolled in the study had prior exposure to daratumumab. Dotted line indicates an ORR of 40% achieved in patients with t(11;14) MM on this study. CI, confidence interval.
Time to progression and duration of response by t(11;14) status. Presented are the time to progression (A) and duration of response (B) data for patients with t(11;14) MM shown in red, and non-t(11;14) MM [includes patients identified as not having t(11;14) or undetermined cytogenetics] shown in blue. The 2 clinical responses in the non-t(11;14) group were reported in 1 patient with translocation of chromosome 14 with an unidentified partner and in a second patient with missing cytogenetics data. Duration of response for these 2 patients in the non-t(11;14) group was 9.5 and 7.2 months, with both still receiving treatment. NE, not evaluable.
Time to progression and duration of response by t(11;14) status. Presented are the time to progression (A) and duration of response (B) data for patients with t(11;14) MM shown in red, and non-t(11;14) MM [includes patients identified as not having t(11;14) or undetermined cytogenetics] shown in blue. The 2 clinical responses in the non-t(11;14) group were reported in 1 patient with translocation of chromosome 14 with an unidentified partner and in a second patient with missing cytogenetics data. Duration of response for these 2 patients in the non-t(11;14) group was 9.5 and 7.2 months, with both still receiving treatment. NE, not evaluable.
For patients with t(11;14) MM, ORRs were apparently not affected by number of prior therapies (supplemental Table 7), refractoriness to prior therapies, or the last therapy (Figure 2B). ORR was 22% (2/9) in patients who had received 1 to 3 prior lines of therapy and 48% (10/21) in those with 4 or more prior lines (supplemental Table 7). In addition, among the 14 patients in the t(11;14) group who achieved clinical benefit (minimum response or better), 3 also had chromosome 17p deletion, 2 had chromosome 13q deletion, and 4 were hyperdiploid (supplemental Table 9).
After disease progression on monotherapy, 17 patients elected to add dexamethasone to venetoclax and remain on study (Figure 1). After adding dexamethasone, 1 patient had PR, 6 had stable disease, 9 progressed, and 1 discontinued before assessment.
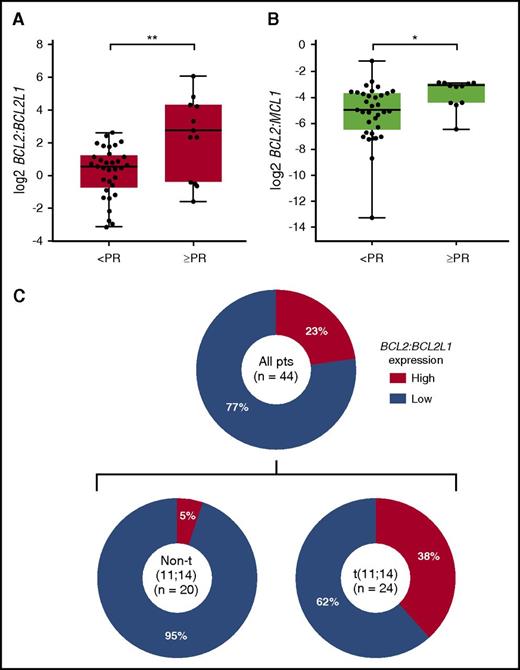
In addition to cytogenetic analyses, baseline bone marrow aspirate samples were available from 47 patients, of which 44 were evaluable for BCL2 (BCL-2), BCL2L1 (BCL-XL), and MCL1 (MCL-1) gene expression by droplet digital polymerase chain reaction (PCR). Gene expression analysis revealed that the ratios of BCL2:BCL2L1 and BCL2:MCL1 were significantly higher in patients who achieved an overall response (≥PR vs < PR) to venetoclax (Figure 4A-B). The rank order of expression profiles that best correlated with response to venetoclax was identified as high BCL2:BCL2L1 > low BCL2L1 > high BCL2:MCL1 > high BCL2 > low MCL1 (supplemental Table 10). On the basis of these results, BATTing19 was used to estimate a threshold value of BCL2:BCL2L1 (log2 ≥ 2.3; P < .01) that would provide optimum selection of patients likely to have a response.
Baseline BCL2:BCL2L1 and BCL2:MCL1 gene expression levels by best response in 44 patients with evaluable bone marrow samples. Quantitation of BCL2, BCL2L1, and MCL1 was performed on CD138-selected BMMCs collected at baseline using droplet digital PCR. Presented are the (A) ratio of BCL2:BCL2L1 and (B) BCL2:MCL1 gene expression levels (log2-transformed copies/µL normalized to housekeeping gene) based on best response (≥PR vs <PR) to venetoclax treatment. Boxes extend from the 25th to the 75th percentile, horizontal bars represent the median, and whiskers extend to the minimum and maximum values. **P < .01; *P < .05 by Wilcoxon rank sum test. (C) Prevalence of high BCL2:BCL2L1 in all patients with evaluable samples (n = 44) and within t(11;14) (n = 24) and non-t(11;14) (n = 20) patients. Bootstrapping and aggregating thresholds from trees (BATTing) was used to estimate a threshold value for the BCL2:BCL2L1 mRNA expression ratio (log2 ≥ 2.3; P < .01) that would provide optimum selection of patients likely to have a response with venetoclax. CD, cluster of differentiation.
Baseline BCL2:BCL2L1 and BCL2:MCL1 gene expression levels by best response in 44 patients with evaluable bone marrow samples. Quantitation of BCL2, BCL2L1, and MCL1 was performed on CD138-selected BMMCs collected at baseline using droplet digital PCR. Presented are the (A) ratio of BCL2:BCL2L1 and (B) BCL2:MCL1 gene expression levels (log2-transformed copies/µL normalized to housekeeping gene) based on best response (≥PR vs <PR) to venetoclax treatment. Boxes extend from the 25th to the 75th percentile, horizontal bars represent the median, and whiskers extend to the minimum and maximum values. **P < .01; *P < .05 by Wilcoxon rank sum test. (C) Prevalence of high BCL2:BCL2L1 in all patients with evaluable samples (n = 44) and within t(11;14) (n = 24) and non-t(11;14) (n = 20) patients. Bootstrapping and aggregating thresholds from trees (BATTing) was used to estimate a threshold value for the BCL2:BCL2L1 mRNA expression ratio (log2 ≥ 2.3; P < .01) that would provide optimum selection of patients likely to have a response with venetoclax. CD, cluster of differentiation.
A high BCL2:BCL2L1 expression ratio was observed in 10/44 (23%) samples, which was enriched in the t(11;14) subgroup [9/24 (38%) evaluable samples from patients with t(11;14) vs 1/20 (5%) in the non-t(11;14) subgroup] (Figure 4C). Eight (80%) of the 10 patients with a high BCL2:BCL2L1 ratio achieved a PR or better (Figure 5A), with a median DOR of 9.7 months; all 8 responders were t(11;14) positive. Median TTP for the 10 patients with a high BCL2:BCL2L1 ratio was 11.5 months (Figure 5C). Three (9%) of 34 patients with a low BCL2:BCL2L1 ratio achieved a PR or better (Figure 5A), with a median DOR of 7.8 months; all 3 responders had t(11;14), and 2 discontinued because of progression, whereas 1 continues receiving venetoclax monotherapy. Median TTP for patients with a low BCL2:BCL2L1 ratio was 1.9 months (Figure 5C). No BCL2 family gene expression profile was available for the 2 responders in the non-t(11;14)/undetermined cytogenetics group. Within t(11;14)-positive patients, 8 (88%) of 9 patients with high BCL2:BCL2L1 expression achieved a PR or better (Figure 5B), with a median DOR of 9.7 months and median TTP of 11.5 months (Figure 5D). The median TTP for t(11;14) patients with low BCL2:BCL2L1 expression (n = 15) was 5.3 months (Figure 5D).
Overall responses and time to progression by BCL2:BCL2L1 gene expression. Presented are the response data for (A) all patients and (B) those with t(11;14) who had evaluable samples for BCL2 and BCL2L1 gene expression by droplet digital PCR. ORR indicates a response of PR or better. Time to progression is shown for (C) all patients and (D) those with t(11;14) who had evaluable samples. Per panel C, median time to progression for 10 patients with a high BCL2:BCL2L1 ratio was 11.5 months. For 34 patients with a low BCL2:BCL2L1 ratio, median time to progression was 1.9 months. All patients, with high or low ratios, who responded to treatment had t(11;14). Per panel D, median time to progression for 9 patients with t(11;14) and a high BCL2:BCL2L1 ratio was 11.5 months. Of 15 patients with t(11;14) and a low BCL2:BCL2L1 ratio, median time to progression was 5.3 months.
Overall responses and time to progression by BCL2:BCL2L1 gene expression. Presented are the response data for (A) all patients and (B) those with t(11;14) who had evaluable samples for BCL2 and BCL2L1 gene expression by droplet digital PCR. ORR indicates a response of PR or better. Time to progression is shown for (C) all patients and (D) those with t(11;14) who had evaluable samples. Per panel C, median time to progression for 10 patients with a high BCL2:BCL2L1 ratio was 11.5 months. For 34 patients with a low BCL2:BCL2L1 ratio, median time to progression was 1.9 months. All patients, with high or low ratios, who responded to treatment had t(11;14). Per panel D, median time to progression for 9 patients with t(11;14) and a high BCL2:BCL2L1 ratio was 11.5 months. Of 15 patients with t(11;14) and a low BCL2:BCL2L1 ratio, median time to progression was 5.3 months.
M-protein data per central laboratory testing were available for 45/66 patients enrolled; patients who discontinued before first central laboratory assessment on cycle 3 day 1 do not have available data for this evaluation. The median best percentage change in primary M-protein for 23 patients with t(11;14) was statistically superior to that for 22 patients without t(11;14) (−53% vs +11%; P < .005 by Wilcoxon rank sum test; supplemental Figure 4). Similarly, the median best percentage change in primary M-protein was also superior in 9 patients with a high BCL2:BCL2L1 ratio compared with that for 21 patients with a low BCL2:BCL2L1 ratio (−98% vs +4%; P < .005; supplemental Figure 4).
Discussion
In this phase 1 study, the BCL-2 inhibitor venetoclax given orally once daily at doses up to 1200 mg was generally safe and well tolerated in patients with relapsed/refractory MM. Most common AEs reported were mild to moderate gastrointestinal toxicities and grade 3/4 hematologic toxicities, which were manageable and did not result in study drug discontinuation. Single-agent venetoclax demonstrated promising antimyeloma activity in patients with t(11;14) MM, which is the most frequent chromosomal translocation in MM and is reported in approximately 15% to 20% of newly diagnosed patients.12,13 The ORR in patients with t(11;14) MM was 40%, and 27% of them had a ≥VGPR response. The responses were durable, with a median DOR of 9.7 months and a median TTP of 6.6 months in the t(11;14) group. In contrast, median TTP was 1.9 months in the non-t(11;14) group. The patients in this study, regardless of t(11;14) status, had a median of 5 prior lines of therapy, with 61% refractory to bortezomib and lenalidomide and 79% refractory to their last prior therapy.
Recent clinical studies with other treatments for MM did not report response rates within the t(11;14) MM subgroup, which makes it challenging to place the current observations in the proper context. However, most trials routinely report response rates for the standard-risk subgroup, which are generally very similar to results in all-comers. In addition, 3 patients in the t(11;14) group treated with venetoclax in the present study also had chromosome 17p deletion and responded to therapy. Although a direct comparison with other studies is not appropriate, the ORR (40%) observed with venetoclax in heavily pretreated t(11;14) relapsed/refractory MM appears encouraging in the context of results seen in all-comers treated with pomalidomide plus dexamethasone (31%-35%),20,21 daratumumab (29%-36%),22,23 or carfilzomib (24%).24 Furthermore, few patients with more advanced disease achieve a ≥VGPR response with these therapies (5%-12%).20,24 Noteworthy, the 27% rate of ≥VGPR responses, as well as the median TTP and median DOR reported in patients in the t(11;14) subgroup treated with venetoclax, were also promising.
The expression of BCL-2 family members in MM cells is heterogeneous, and upregulation of either MCL-1 or BCL-XL by these cells can confer resistance to venetoclax.25 Accordingly, human myeloma cell lines sensitive to venetoclax alone had higher expression of BCL-2 and lower levels of BCL-XL and MCL-1.11,25 In this clinical study, an ORR of 80% and a median TTP of 11.5 months was observed among 10 patients with a high BCL2:BCL2L1 gene expression ratio treated with venetoclax monotherapy. As expected, this favorable BCL-2 profile was more frequent in the t(11;14) than in the non-t(11;14) subgroup (38% vs 5%, respectively).
Other antimyeloma therapies can also modulate BCL-2 profile and enhance the sensitivity of myeloma cells to venetoclax. Proteasome inhibitors such as bortezomib and carfilzomib can upregulate the MCL-1 inhibitor NOXA, which leads to functional neutralization of MCL-1 in human myeloma cell lines.26,27 Dexamethasone also increases BCL-2 dependency of myeloma cells by upregulating the expression of the proapoptotic molecule BIM and shifting its binding toward BCL-2, resulting in increased sensitivity to venetoclax.28 Accordingly, an ongoing phase 1b study of venetoclax in combination with bortezomib and dexamethasone (NCT01794507) has shown high response rates in patients with relapsed/refractory MM irrespective of t(11;14) status.29 In the present study, the addition of dexamethasone at the time of progression did not appear to add any clinical benefit for patients on venetoclax monotherapy.
MM is a complex disease with high genetic variability.30,31 Advances in understanding MM biology as well as the development of novel agents have increased clinical response rates and prolonged patient survival, although MM is still considered incurable for most patients. Despite many advances, at this time, there are no biologic-based therapeutic approaches in the management of MM. Given the multiple available drugs and combination regimens in this disease, predictive markers to guide selection of therapy would be a key advancement in the care of these patients, delivering the most effective treatments to the right patients.32 The findings of the current study are commensurate with preclinical findings and raise the possibility that future myeloma therapy may be driven by the underlying genetic abnormality or another surrogate biomarker.
In conclusion, the oral BCL-2 inhibitor venetoclax at a daily dose up to 1200 mg demonstrated single-agent antimyeloma activity in patients with relapsed/refractory MM positive for t(11;14), for whom multiple prior lines of therapy have failed. Venetoclax has a unique mechanism of action and may offer a novel biologic-driven approach in MM.
Presented in part during the annual meetings of the American Society of Clinical Oncology (ASCO), Chicago, IL, 29 May 2015; European Hematology Association (EHA), Vienna, Austria, 13 June 2015 (encore of ASCO 2015 presentation); International Conference on Malignant Lymphoma (ICML), Lugano, Switzerland, 17 June 2015; International Myeloma Workshop (IMW), Rome, Italy, 23 September 2015 (encore of ASCO 2015 presentation); American Society of Hematology (ASH), Orlando, FL, 5 December 2015; ASCO, Chicago, IL, 6 June 2016; EHA, Copenhagen, Denmark, 12 June 2016 (encore of ASCO 2016 presentation); Pan Pacific Lymphoma Conference (PPLC), Koloa, HI, 18 July 2016 (encore of ASCO 2016 presentation); ASH, San Diego, CA, 4 December 2016; IMW, New Delhi, India, 4 March 2017 (encore of ASH 2016 presentation).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Special thanks to the patients and their families, study coordinators, and support staff. Medical writing support was provided by Sharanya Ford. Data programming support was provided by Ruiling Zhang and Ming Zhu. Biomarker analysis support was provided by Yan Sun. All are employees of AbbVie.
Venetoclax is being developed in collaboration between AbbVie and Genentech. AbbVie and Genentech provided financial support for the study and participated in the design, study conduct, analysis and interpretation of data, as well as the writing, review, and approval of the manuscript. This study was supported by research funding from AbbVie and Genentech to S.K., J.L.K., C.G., J.M., R.V., B.P., L.B., T.F., M.A., P.M., and C.T.
Authorship
Contribution: S.K., M.A, P.M., E.A.P., S.H.E., J.D.L., J.A.R., P.C.M., M.V., and C.T. designed research; S.K., J.L.K., C.G., J.M., R.V., B.P., L.B., T.F., M.A., P.M., J.A.R., P.C.M., and C.T. collected data; and S.K., J.L.K., C.G., J.M., R.V., B.P., L.B., T.F., M.A., P.M., E.A.P., S.A., M.D., T.X., S.K.A., S.H.E., J.D.L., J.A.R., P.C.M., M.V., and C.T. analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: S.K. received research support to the Mayo Clinic for clinical trials from Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, and Novartis; was a consultant (with no personal compensation) to Celgene, Millennium, BMS, Onyx, Janssen, and Noxxon; and received an honorarium from Skyline. J.L.K. was a consultant for Amgen, Roche, BMS, Seattle Genetics, Sutro Biopharma, and Pharmacyclics and received research support from Amgen and Novartis. C.G. received honoraria from Janssen, BMS, and Celgene; was a consultant for Janssen, BMS, and Celgene; received research support from Celgene; and received travel, accommodations, or other expenses paid or reimbursed by Janssen, BMS, and Celgene. J.M. received institutional research funding from Onyx, Celgene, Sanofi, and AbbVie. R.V. was a consultant for Celgene, Onyx, Takeda, Novartis, BMS, Sanofi, Janssen, and Merck and received research support from Takeda and Onyx. B.P. was a consultant for Takeda, Novartis, Janssen, and BMS. L.B. was a consultant for Takeda, Celgene, Janssen, and Amgen. P.M. was an advisory board member for Celgene, Janssen, Takeda, Novartis, and Amgen. E.A.P. is a Genentech employee and owns stock. C.T. received research funding from AbbVie. S. A., M.D., T.X., S.K.A., S.H.E., J.D.L., J.A.R., P.C.M., and M.V. are AbbVie employees and own stock. The remaining authors declare no competing financial interests.
Correspondence: Shaji Kumar, MD, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.


![Figure 3. Time to progression and duration of response by t(11;14) status. Presented are the time to progression (A) and duration of response (B) data for patients with t(11;14) MM shown in red, and non-t(11;14) MM [includes patients identified as not having t(11;14) or undetermined cytogenetics] shown in blue. The 2 clinical responses in the non-t(11;14) group were reported in 1 patient with translocation of chromosome 14 with an unidentified partner and in a second patient with missing cytogenetics data. Duration of response for these 2 patients in the non-t(11;14) group was 9.5 and 7.2 months, with both still receiving treatment. NE, not evaluable.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/22/10.1182_blood-2017-06-788786/4/m_blood788786f3.jpeg?Expires=1765894016&Signature=lDWjkF5rmm4b1qXrgksWnzv7UnpP3PFrvNhSpGVmCIYssHnUJhOkv8h1ZIYQhq2tlc3kZoe54VArfY0ekQtsJSGo3yTky46R4Ofdtemo2kccoY4w0Ys4mh6Ao3XOsa7oAttPhOJzV1C1Y0GQ-KIjDJrcMTV4J6lbjnCoB1JSvm-6Lrm9ktdiwbFElI7CHna3E9g8V27F2r2vySIC2paRrvDeqgp2sF5PFa4aMpCRoLfUpHdNrIerD5BjsPlaRXpFJj7NBQ4QHZs8~K8IpZk94Qjdjhj3jvFSZ2ym8Dr-OQ9P4UTGktKMlzwnQhCxY8BQuHO7QIk3Yv4IUcbJkm5Lgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal