Abstract
The optimal duration of anticoagulant therapy in patients with cancer-associated venous thromboembolism (VTE) is unknown. Without well-designed studies evaluating the efficacy, safety, and cost-effectiveness of continuing anticoagulant therapy beyond the acute treatment period of 3 to 6 months, evidence-based recommendations are lacking. Consensus guidelines generally suggest continuing anticoagulation treatment in patients with active cancer or receiving cancer treatment, with periodic reassessment of the risks and benefits. Unfortunately, with very little published data on the epidemiology of cancer-associated VTE beyond the initial 6 months, it is not possible for clinicians and patients to weigh risks and benefits in a quantitatively informed manner. Further research is needed to provide reliable and contemporary estimates on the risk of recurrent VTE off anticoagulant therapy, risk of bleeding on anticoagulant therapy, case fatality or all-cause mortality, and other important consequences of living with cancer-associated VTE. This chapter provides an overview of the published literature on real-world data on anticoagulant therapy use, the risks and risk factors of recurrent VTE and bleeding, and patient preference and values regarding long-term anticoagulation treatment. It will conclude with a pragmatic, experience-informed approach for tailoring anticoagulant therapy in patients with cancer-associated VTE.
Introduction
Patients living with cancer experience many challenges. One of the most common consequences of both cancer and its treatments is venous thromboembolism (VTE). Not only is it associated with shortened patient survival, it also has a negative impact on quality of life because many patients suffer chronic, residual symptoms and experience distress and anxiety from fear of recurrence. Treatment of cancer-associated VTE is also burdensome; patients receiving low-molecular-weight heparin must endure daily subcutaneous injections and often face financial hardship, and those taking vitamin K antagonist (VKA) therapy need frequent laboratory monitoring, dose adjustments, and modifications of their diet and lifestyle. Anticoagulant-related bleeding further complicates management. Consequently, physicians and patients invariably struggle with the decision whether to stop or continue anticoagulation after acute treatment of the index thrombotic event. Unfortunately, few data have been published to help physicians and patients make this decision. Consensus guidelines generally suggest continuing anticoagulant therapy in patients with active cancer or receiving cancer treatment, with periodic reassessment of the risks and benefits. Unfortunately, with a paucity of published information on the epidemiology of cancer-associated VTE beyond the initial 6 months, it is not possible for physicians and patients to weigh risks and benefits in a quantitatively informed manner.
To address the optimal duration of anticoagulation, I will review the published literature on real-world data regarding anticoagulant therapy use, the risks and risk factors of recurrent VTE and bleeding, and patient preference and values regarding long-term anticoagulation treatment. To conclude, I will outline how I approach this personalized discussion with my patients.
The real-world data on anticoagulant therapy utilization
Real-world data reporting has become increasingly prevalent. In the setting of cancer-associated VTE, large administrative databases, primarily from health care insurance claims, have been mined for information. Although these studies are impressive in terms of the quantity of data included, there are quality limitations of such retrospective analyses of administrative data in terms of the accuracy, consistency, and completeness of coding and reporting. Inherent but hidden bias also arises from patient and therapeutic selection because of the type of health care plan, extent of coverage, and access to care. Even if prescribed-medication data are sometimes available, information on adherence and association with clinical events is missing. Adjustment for confounding and competing factors is also challenging. Nonetheless, such observational data do provide a useful overview of the patterns of practice and prevalence of clinical outcomes outside of the clinical trial setting.
A recent retrospective analysis used the Humedica database to inform real-world clinical practice and patient outcomes in patients with cancer and thrombosis.1 This massive health information technology database houses longitudinal individual patient data throughout the United States and from different health plan types, including commercial, Medicaid, and Medicare. In this study, data between July 2007 and March 2014 from 72 224 adult patients with history of cancer and thrombosis were included. In this analysis, there were 8222 patients with active cancer, defined as those with International Classification of Diseases-9 codes for cancer diagnosis and cancer treatment captured within 6 months. Disturbingly, 28% of these patients were not treated with any anticoagulant therapy, 26% received parenteral therapy only, and the remainder received either an oral anticoagulant alone (14%) or parenteral plus oral therapy (32%). Furthermore, the mean duration of parenteral therapy was only 1.3 months whereas oral anticoagulant therapy was given for 2.8 months.
Duration of anticoagulant use is also reported by Kaatz et al.2 Extracting data between June 2007 and September 2011 from the HealthCare Integrated Research Database, which contains data from a large managed-care organization that serves approximately 14 million Americans, 2002 patients with new VTE with a minimal of 1 year of follow-up were identified. Patients with cancer-associated VTE accounted for 16.4% of the cohort, and their mean duration of treatment was 297 days (standard deviation ± 271); 89% were treated with warfarin. Also, patients rated as having a high or intermediate risk of bleeding were less likely to discontinue than those with a low bleeding risk. This finding remained even after adjustment for the risk of VTE recurrence. Further exploration of the data to understand this unexpected observation was limited by the nature of the dataset.
Overall, published retrospective studies, surveys, and registries conducted from around the world uniformly report suboptimal adherence to guideline-recommended therapy even during the acute treatment period.3 Reasons for this poor performance are unknown.
Bleeding: risk and risk factors
The most serious adverse effect of anticoagulation is bleeding. Although anticoagulants do not cause bleeding, they intensify the severity of any bleeding by interfering with hemostasis. Consequently, cancer patients have a higher risk of bleeding than noncancer patients do because they are uniquely at risk of malignant vascular or mucosal invasion and cancer- or chemotherapy-induced thrombocytopenia. They also have a high prevalence of comorbidities commonly associated with bleeding, including older age, frailty, renal impairment, and liver dysfunction.
Similar to those in patients without cancer, the most common sites of bleeding in patients with malignancy are the gastrointestinal and genitourinary tracts.4-6 However, unlike patients without cancer, bleeding events in cancer patients do not correlate with the intensity of anticoagulation in older studies using warfarin.4,7 This finding may reflect that bleeding is exacerbated at tumor sites even at a low intensity of anticoagulation.
In the real-world setting, bleeding is likely more prevalent than in clinical trials with carefully selected patients. In the study using the Humedica database, active cancer patients with thrombosis had an incidence rate of 31.2 per 100 person-years for major bleeding during follow-up.1 Major bleeding was defined as any bleeding event that resulted in hospital admission or a blood transfusion. No information is available on the timing of events or if bleeding occurred during anticoagulant treatment. Clinical factors associated with increased risk of major bleeding included age ≥65 years, heart disease, heart failure, renal disease, hepatic disease, peripheral arterial disease, diabetes, hypertension, hemorrhagic stroke, prior major bleeding, prior fracture/trauma, emergency room visits, and hospitalization. These risk factors have been identified previously in various bleeding predictive models in VTE and non-VTE settings.8,9
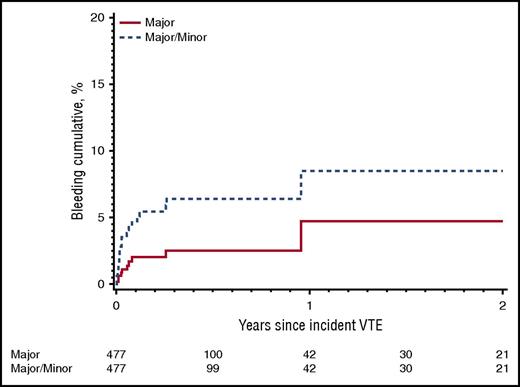
Using the population-based Rochester Epidemiology Project database from the Mayo Clinic that followed all Olmsted County residents from 1966 to 2000, Chee et al reported the cumulative risk of major bleeding was 4.0% at 1 year, adjusted for death, for patients with cancer-associated VTE treated with warfarin.10 Most of the bleeding events occurred early during the first 3 months of treatment. Thereafter, the incidence of major bleeding stabilizes at approximately 0.2% per month (Figure 1).
Cumulative incidence of first major bleeding event and first major or minor bleeding event while receiving anticoagulation therapy among Olmsted County, Minnesota, residents with incident active cancer-associated VTE, 1966-2000, and followed-up through 31 December 2005.10 Reprinted from Chee et al10 with permission.
Cumulative incidence of first major bleeding event and first major or minor bleeding event while receiving anticoagulation therapy among Olmsted County, Minnesota, residents with incident active cancer-associated VTE, 1966-2000, and followed-up through 31 December 2005.10 Reprinted from Chee et al10 with permission.
The Registro Informatizado de Enfermedad TromboEmbólic (RIETE) registry also provides real-world estimates of the incidence of outcome events after a diagnosis of symptomatic deep vein thrombosis (DVT) or pulmonary embolism (PE). Since 2001, this ongoing, international registry has been collecting prospective data from consecutive patients. In a report that included data up to May 2007 of 3805 cancer patients, 156 (4.1%) had major bleeding during the first 3 months of treatment, but there was no information on the incidence beyond 3 months.5 On multivariate analysis, recent major bleeding, creatinine clearance <30 mL/min, immobility for ≥4 days, or metastatic disease had odds ratios ranging from 1.6 to 2.4 for major bleeding (Table 1). In another RIETE publication, the risk of major bleeding varied according to tumor type.11
Factors previously reported to be independently associated with increased risk of major bleeding in patients with cancer-associated VTE
| Study . | Clinical factor or biomarker . | Estimated risk (95% CI) . |
|---|---|---|
| Prandoni et al, 20024 | Genitourinary cancer* | HR: 4.5 (2.1-9.9)† |
| Extensive cancer | HR: 4.8 (2.3-10.1)† | |
| Trujillo-Santos et al, 20085 | Metastatic cancer | OR: 1.6 (1.1-2.3) |
| Immobility ≥ 4 d | OR: 1.8 (1.2-2.7) | |
| Creatinine clearance < 30 mL/min | OR: 2.2 (1.5-3.4) | |
| Recent major bleeding | OR: 2.4 (1.1-5.1) | |
| Kamphuisen et al, 201515 | Metastatic cancer | RR: 1.6 (1.1-2.3) |
| Intracranial lesion | RR: 2.0 (1.1-3.5) | |
| Age > 75 y | RR: 1.8 (1.2-2.7) | |
| Mahé et al, 201711‡ | Lung cancer | HR: 1.8 (1.1-3.0)‡ |
| Colorectal cancer | HR 2.1 (1.3-3.4)‡ | |
| Prostate cancer | HR: 2.1 (1.3-3.5)‡ | |
| Cancer diagnosis within past 3 mo | HR: 1.6 (1.1-2.3) | |
| Platelet count < 100 × 109/L | HR: 2.0 (1.4-3.0) | |
| Recent major bleeding | HR: 5.0 (3.1-7.9) |
| Study . | Clinical factor or biomarker . | Estimated risk (95% CI) . |
|---|---|---|
| Prandoni et al, 20024 | Genitourinary cancer* | HR: 4.5 (2.1-9.9)† |
| Extensive cancer | HR: 4.8 (2.3-10.1)† | |
| Trujillo-Santos et al, 20085 | Metastatic cancer | OR: 1.6 (1.1-2.3) |
| Immobility ≥ 4 d | OR: 1.8 (1.2-2.7) | |
| Creatinine clearance < 30 mL/min | OR: 2.2 (1.5-3.4) | |
| Recent major bleeding | OR: 2.4 (1.1-5.1) | |
| Kamphuisen et al, 201515 | Metastatic cancer | RR: 1.6 (1.1-2.3) |
| Intracranial lesion | RR: 2.0 (1.1-3.5) | |
| Age > 75 y | RR: 1.8 (1.2-2.7) | |
| Mahé et al, 201711‡ | Lung cancer | HR: 1.8 (1.1-3.0)‡ |
| Colorectal cancer | HR 2.1 (1.3-3.4)‡ | |
| Prostate cancer | HR: 2.1 (1.3-3.5)‡ | |
| Cancer diagnosis within past 3 mo | HR: 1.6 (1.1-2.3) | |
| Platelet count < 100 × 109/L | HR: 2.0 (1.4-3.0) | |
| Recent major bleeding | HR: 5.0 (3.1-7.9) |
HR, hazard ratio; OR, odds ratio; RR, relative risk.
Uterus, kidney, ovary or testicle, bladder, and prostate.
Compared with patients without cancer.
Study included only patients with breast, prostate, colorectal, or lung cancer. Risk is relative to patients with breast cancer.
From randomized controlled trials that exclusively included cancer patients, the incidence of major bleeding ranged from 4.6% to 11.6% during the first 3 to 6 months of anticoagulation treatment.12 The risk of major bleeding is comparable between low-molecular-weight heparin and VKA therapy.13 In the most recent international trial (Comparison of Acute Treatments in Cancer Haemostasis [CATCH]) with 900 cancer patients, 2.7% of patients given tinzaparin and 2.4% of patients treated with warfarin developed major bleeding, whereas 10.9% and 15.3%, respectively, developed clinically relevant nonmajor bleeding.14 Age >75 years, metastatic disease, and having an intracranial malignant lesion (primary or secondary) were associated with clinically relevant bleeding (Table 1).15
The DALTECAN study provided prospective data on the risk of bleeding during the first 12 months of low-molecular-weight heparin treatment in cancer patients.6 The primary outcome of the study was the rate of major bleeding between 6 and 12 months of treatment with dalteparin. A total of 334 patients with active cancer and acute VTE were treated with dalteparin following the CLOT regimen, followed by continuation of the maintenance dose of approximately 150 U/kg once daily for up to 12 months. The mean duration of treatment was 210 days, with 109 of the 334 patients (33%) completing 12 months of dalteparin injections. The most common reason for drug discontinuation was death related to cancer. In the first month of treatment, 3.6% of patients had major bleeding. During months 2 to 6, the incidence was 1.1% per patient-month and during months 7 to 12, the incidence was 0.7% per patient-month. The difference in major bleeding rates between these 2 time periods was not statistically significant.
Taking into consideration all the methodological limitations of published data, current evidence does demonstrate that the risk of clinically important bleeding with anticoagulation is highest during the first month of therapy but is lower thereafter at approximately 0.5% per month during anticoagulant therapy. The risk appears to be independent of the type of anticoagulant and the intensity of anticoagulation but varies with patient- and cancer-specific comorbidities. There are no published studies of biomarkers for predicting bleeding in patients with cancer and VTE.
Recurrent thrombosis: risk and risk factors
The incidence of recurrent VTE is high in patients with active cancer. In the Olmsted County population from 1988 to 2000, the estimated 5-year VTE recurrence rate in patients with cancer-associated VTE was 43.4%.16 Adjusted for the competing risk of death, the rate remains high at 33.8%. This is substantially higher than in idiopathic and noncancer secondary VTE, where the rates were 26.2% and 16.8%, respectively. The risk is also high despite anticoagulation treatment. In a prospective cohort study with 181 cancer patients therapeutically anticoagulated with warfarin, 20.7% developed recurrent VTE over 12 months of follow-up.4 In a retrospective analysis of randomized controlled trials, Hutten et al reported a recurrent VTE incidence of 13.3% per patient-year in 264 cancer patients treated with warfarin.7 A much higher incidence was observed with subtherapeutic values of the international normalized ratio (INR), but recurrent VTE still occurs with therapeutic INR levels. These results are consistent with data from randomized controlled trials that demonstrated poor efficacy with VKA therapy compared with low-molecular-weight heparin.12-14 In the CLOT and CATCH trials, the 6-month risk of symptomatic, recurrent VTE in those treated with VKA therapy was 12% and 10.5%, respectively, with the INR time-in-therapeutic range at 46% and 47%, respectively.15,17,18 Altogether, the literature confirms what is well known by clinicians treating cancer patients with VTE: maintaining the INR within the therapeutic range is difficult in cancer patients, and recurrent VTE occurs despite therapeutic VKA therapy. This high failure rate, combined with the nuisance of frequent laboratory monitoring, dietary restrictions, and drug interactions, makes VKA an undesirable option for treatment and secondary prophylaxis of cancer-associated VTE.
Although treatment with low-molecular-weight heparin is the guideline-recommended regimen of choice, recurrent VTE still occurs.19-21 In the CLOT and CATCH trials, the 6-month incidence of symptomatic, recurrent VTE in the low-molecular-weight heparin groups was 6% and 7.2%, respectively.15,17,18 The DALTECAN study further showed the cumulative probability of recurrent VTE was 9% and 14% at 6 and 12 months, respectively.6 The risk was highest in the first month at 5.7%, dropping down to 3.4% during months 2 to 6 and 4.1% during months 7 to 12.
But outcome event rates in rigorously controlled clinical trials are often not replicated in the real-world setting. Patient selection, ascertainment of outcomes, adherence with treatment, comorbidities, and competing factors may account for the differences. In the Humedica database from 2007 to 2014, active cancer patients with thrombosis had a VTE recurrence incidence rate of 24.7 per 100 person-years.1 For Olmstead county residents with active cancer-associated VTE between 1966 and 2000, the cumulative incidence of VTE recurrence adjusted for competing risk of death was 19.6% at 1 year, 21.9% at 2 years, 26.6% at 5 years, and 28.6% at 10 years.10 From the RIETE registry from 2001 to 2007, 5.0% of patients with active cancer had recurrent DVT or PE during the first 3 months of treatment.5 These nonclinical trial observations should be interpreted with caution given the age of the data and the potential impact of modern cancer treatment on the risk of thrombosis, bleeding, and mortality.
Unfortunately, knowing the risk of recurrent VTE during anticoagulant therapy offers limited value in deciding on when it is safe to stop anticoagulation. To date, there are no published data on the incidence of recurrent VTE in patients with cancer after completing a defined course of anticoagulation treatment. An indirect estimate of the risk of recurrent VTE after anticoagulation is discontinued comes from the Rochester Epidemiology Project. In this population-based study, the VTE recurrence per 100 person-years was 12.8 from month 6 to 12, 6.1 from year 1 to 2, 5.8 from year 2 to 5, and 1.7 from year 5 to 10.10 These patients had received a median duration of warfarin anticoagulation treatment of 79 days (interquartile range, 18-166), so the vast majority had stopped warfarin by 6 months.
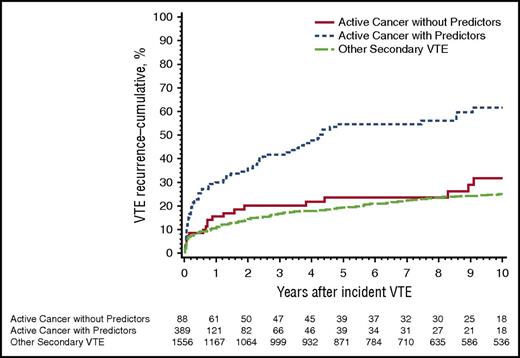
A number of clinical features have been identified to correlate with a higher risk of recurrent cancer-associated VTE (Table 2).22 Metastatic or extensive malignant disease has a two- to threefold higher risk of recurrent VTE than earlier stages of cancer.4,10,18,23 Lung cancer is associated with a higher risk, whereas breast cancer is associated with a lower risk.4,11,23 A multivariate analysis identified an increased hazard with one or more predictors, including stage IV cancer (particularly pancreatic cancer); brain, lung and ovarian cancer; myeloproliferative or myelodysplastic disorder; cancer progression; and neurological disease with leg paresis (Figure 2).10 Chemotherapy was not a significant predictor, but certain chemotherapeutic agents or regimens might be highly thrombogenic.24,25 From the RIETE registry, a multivariate analysis found that age <65 years, symptomatic PE as the index event, and diagnosis of cancer less than 3 months prior to VTE were significantly associated with recurrent VTE during the first 3 months of treatment (Table 2).5 From the CATCH trial, hepatobiliary cancers and venous compression secondary to tumor or malignant adenopathy were found to be independent risk factors for recurrent VTE.26 Risk models for predicting recurrent VTE have incorporated some of these features, but they need further validation.22
Factors previously reported to be independently associated with increased risk of recurrent VTE in patients with cancer-associated VTE
| Study . | Clinical factor or biomarker . | Estimated risk (95% CI) . |
|---|---|---|
| Prandoni et al, 20024 | Extensive cancer | HR: 4.6 (2.3-9.0)* |
| Lung cancer | HR: 6.9 (3.0-15.9)* | |
| Gastrointestinal cancer† | HR: 5.1 (2.3-11.3)* | |
| Genitourinary cancer‡ | HR: 3.7 (1.7-8.0)* | |
| Trujillo-Santos et al, 20085 | Age < 65 y | OR: 3.0 (1.9-4.9) for rPE |
| OR: 1.6 (1.0-2.4) for rDVT | ||
| Diagnosis < 3 mo earlier | OR: 2.0 (1.2-3.2) for rPE | |
| OR: 2.4 (1.5-3.6) for rDVT | ||
| Clinically overt PE | OR: 1.9 (1.2-3.1) | |
| Louzada et al, 201123 | Metastatic cancer | RR: 1.36 (1.06-1.74) |
| Chee et al, 201410 | Stage IV pancreatic cancer | HR: 6.38 (2.69-15.13) |
| Brain cancer | HR: 4.57 (2.07-10.09) | |
| MPN or MDS | HR: 3.49 (1.59-7.68) | |
| Ovarian cancer | HR: 3.22 (1.57-6.59) | |
| Stage IV (nonpancreas) cancer | HR: 2.85 (1.74-4.67) | |
| Lung cancer | HR: 2.73 (1.63-4.55) | |
| Neurological disease with leg paresis | HR: 2.38 (1.14-4.97) | |
| Cancer stage progression | HR: 2.14 (1.30-3.52) | |
| Mahé et al, 201711 | Lung cancer | HR: 3.8 (2.6-5.6)§ |
| Khorana et al, 201726 | Hepatobiliary cancer | sHR: 5.5 (2.3-13.6) |
| Venous compression | sHR: 3.1 (1.4-6.5) | |
| Tissue factor antigen | sHR: 3.3 (1.7-6.4) | |
| C-reactive protein | sHR: 1.9 (0.97-3.8) |
| Study . | Clinical factor or biomarker . | Estimated risk (95% CI) . |
|---|---|---|
| Prandoni et al, 20024 | Extensive cancer | HR: 4.6 (2.3-9.0)* |
| Lung cancer | HR: 6.9 (3.0-15.9)* | |
| Gastrointestinal cancer† | HR: 5.1 (2.3-11.3)* | |
| Genitourinary cancer‡ | HR: 3.7 (1.7-8.0)* | |
| Trujillo-Santos et al, 20085 | Age < 65 y | OR: 3.0 (1.9-4.9) for rPE |
| OR: 1.6 (1.0-2.4) for rDVT | ||
| Diagnosis < 3 mo earlier | OR: 2.0 (1.2-3.2) for rPE | |
| OR: 2.4 (1.5-3.6) for rDVT | ||
| Clinically overt PE | OR: 1.9 (1.2-3.1) | |
| Louzada et al, 201123 | Metastatic cancer | RR: 1.36 (1.06-1.74) |
| Chee et al, 201410 | Stage IV pancreatic cancer | HR: 6.38 (2.69-15.13) |
| Brain cancer | HR: 4.57 (2.07-10.09) | |
| MPN or MDS | HR: 3.49 (1.59-7.68) | |
| Ovarian cancer | HR: 3.22 (1.57-6.59) | |
| Stage IV (nonpancreas) cancer | HR: 2.85 (1.74-4.67) | |
| Lung cancer | HR: 2.73 (1.63-4.55) | |
| Neurological disease with leg paresis | HR: 2.38 (1.14-4.97) | |
| Cancer stage progression | HR: 2.14 (1.30-3.52) | |
| Mahé et al, 201711 | Lung cancer | HR: 3.8 (2.6-5.6)§ |
| Khorana et al, 201726 | Hepatobiliary cancer | sHR: 5.5 (2.3-13.6) |
| Venous compression | sHR: 3.1 (1.4-6.5) | |
| Tissue factor antigen | sHR: 3.3 (1.7-6.4) | |
| C-reactive protein | sHR: 1.9 (0.97-3.8) |
HR, hazard ratio; MPN, myeloproliferative neoplasm; MDS, myelodysplastic syndrome; OR, odds ratio; RR, relative risk; sHR, subdistrubutional hazard ratio; rDVT, recurrent deep vein thrombosis; rPE, recurrent pulmonary embolism.
Compared with patients without cancer.
Colorectal, stomach or esophagus, pancreas, liver, or gallbladder.
Uterus, kidney, ovary or testicle, bladder, prostate.
Compared with patients with breast cancer.
Cumulative incidence of first VTE recurrence among Olmsted County, Minnesota, residents with incident DVT or PE, 1966-2000, associated with active cancer and one or more predictor of VTE recurrence, active cancer and no predictor, and noncancer secondary VTE.10 Reprinted from Chee et al10 with permission.
Cumulative incidence of first VTE recurrence among Olmsted County, Minnesota, residents with incident DVT or PE, 1966-2000, associated with active cancer and one or more predictor of VTE recurrence, active cancer and no predictor, and noncancer secondary VTE.10 Reprinted from Chee et al10 with permission.
More recently, studies have focused on identifying biomarkers that can predict recurrent VTE (Table 2). For cancer-associated VTE, D-dimer, prothrombin fragment 1+2, soluble P-selectin, and tissue factor appear to influence the risk for a first episode of VTE during the first year after cancer diagnosis.27 However, a preplanned regression analysis of the CATCH trial did not find an association between baseline levels of D-dimer, factor VIII, or soluble P-selectin at VTE diagnosis and recurrent VTE, but the highest quartile of circulating tissue factor antigen level was independently associated with a threefold risk of recurrence.26
Overall, the risk of recurrent VTE in patients with active cancer is substantial both during and after anticoagulation treatment. Clinical factors associated with recurrence included metastatic disease, certain tumor types, and venous stasis (from venous compression or leg paresis). High tissue factor level at the time of VTE diagnosis is predictive of recurrence during anticoagulant therapy.
Mortality and case fatality: the body count
Historically, up to half of cancer patients have autopsy evidence of PE at the time of death.28 The majority of cases were unrecognized antemortem, with 30% of VTE cases with active cancer diagnosed solely at autopsy.10 Many patients who died of massive PE had localized or limited malignant disease.29
In the modern era, VTE remains a leading cause of death in patients with cancer.30 Mortality after cancer-associated VTE is high. In prospective and randomized trials, 6-month mortality is approximately 35%.6,14,17,31 In a Canadian population study using administrative health care databases, the 1-year survival rate for cancer patients with an index VTE between January 2000 and December 2009 was 0.47 (95% CI, 0.46-0.48).32 This is much lower than the survival rate of 0.93 for cases with unprovoked VTE and 0.84 for cases with VTE and a noncancer major risk factor. In the EPIPHANY observational study of 1033 cancer patients, the overall 30-day and 90-day mortality was 21% and 33%, respectively, for those with symptomatic PE. Prior VTE was an independent predictor of death (odds ratio, 2.2; 95% CI, 1.2-3.9).33
Survival is even worse for cancer patients who develop recurrent VTE and anticoagulant-related bleeding. In the Olmsted County cohort of active cancer patients, the cumulative mortality at 90 days was 67.2% for those with recurrent PE with or without DVT and was 30.7% for those with recurrent DVT alone.10 Adjusting for age, sex, and predictors of VTE recurrence, recurrent VTE increased the hazard of death almost threefold (hazard ratio, 2.7; 95% CI 2.1-3.4). Bleeding also increased the risk of death by more than twofold (hazard ratio, 2.3; 95% CI 1.5-3.5).
Death as a result of recurrent VTE and death due to major bleeding in cancer patients are much higher than in noncancer patients. In prospective studies primarily of patients without cancer, Carrier et al reported a case fatality of 11.3% for recurrent VTE and 11.3% for major bleeding during the first 3 months of anticoagulation treatment and a case fatality of 3.6% for recurrent VTE after anticoagulation treatment.34 In a prospective study of patients diagnosed with cancer-associated VTE between 2004 and 2014, the case fatality for was 46.7% for recurrent VTE and 23.3% for major bleeding during low-molecular-weight heparin treatment.35 However, the respective rates in the DALTECAN study were only 10.8% and 5.9%.6 In the CLOT and CATCH trials, case fatality for recurrent VTE was 15.0% and 44.7%, respectively, and for major bleeding was 3.2% and 26.1%, respectively.15,17 The great variability in these rates across studies reflects the heterogeneity of the study designs, patient populations, anticoagulant treatments, definitions of fatal PE, and details of follow-up. Additional reasons for the differences observed in CLOT and CATCH trials may include variations in the adjudication processes, the diverse management of thrombosis and bleeding around the world, and the decade difference between when these trials were conducted. Unfortunately, what remains unknown is the case fatality of recurrent VTE without anticoagulant therapy after the acute treatment period and of major bleeding if anticoagulation therapy is continued after the acute treatment period. These are critical estimates for balancing the fatal consequences of thrombosis vs bleeding when making a decision on whether to stop or continue anticoagulation treatment.
Patient preference and values
Qualitative literature has given us new insight on patient preference and values regarding anticoagulant therapy. Uniformly, patients living with cancer find VTE a distressing and unpleasant experience. This may be a result of being poorly informed about this potential complication in their cancer journey, overwhelmed by the complexity of care, and fearful about how this complication may impact their cancer treatment and prognosis.36 Consequently, avoidance of first and recurrent episodes of VTE is important in their overall goals of care.
Studies in cancer-associated VTE have also shown that daily low-molecular-weight heparin self-injection therapy is feasible. In clinical trials, adherence with daily injections for up to 12 months is as high as 95%.6 Arguably, study volunteers are motivated and receive extensive support and education, but even outside the clinical trials setting, injection treatment is acceptable to patients. A prospective study in The Netherlands reported that low-molecular-weight heparin was stopped after a median duration of 90 days, whereas a study in France observed that patients experienced a high degree of satisfaction with 6 months of daily injections.35,37 Indeed, patients report greater acceptability of low-molecular-weight heparin injections than other cancer-related treatments, such as surgery and chemotherapy.38 In contrast, warfarin is associated with a reduced quality of life because of the uncertainty of dosing and need for laboratory monitoring.38 The oral route of administration is rated as less important than receiving an anticoagulant regimen that does not interfere with their cancer treatment and that provides maximum efficacy and acceptable safety.39
On the other hand, physicians tend to underestimate a patient’s capability to accept long-term daily injections.40 This is reflected in the poor adherence to guideline recommendations and underutilization of low-molecular-weight heparin based on registry reports and surveys.3 Reported practices from administrative databases provide an even more alarming picture of the management of cancer-associated VTE in the real world.1,2 The detrimental impact of suboptimal treatment, especially during the first 3 to 6 months of therapy, of cancer-associated VTE on quality of life and survival has yet to be determined.
When to stop anticoagulation treatment?
In the noncancer setting, the optimal duration of anticoagulation treatment has been extensively investigated, and clear recommendations are available from evidence-based guidelines.19 In the cancer setting, comparable quality evidence is lacking. Available data are largely outdated and selective, and advances in cancer treatment have had a significant impact on the risk of treatment-related thrombosis and bleeding as well as mortality. Nonetheless, data within studies do suggest that the absolute risk of recurrent VTE remains higher than the risk of major bleeding even after 6 months of anticoagulation treatment. In the Rochester Epidemiology Project database, the adjusted cumulative risks of recurrence vs major bleeding are 16.6% vs 2.0% at 6 months and 19.6% vs 4.0% at 1 year.10 Based on these estimates, the case fatality of bleeding must be at least five- to eightfold higher than that of recurrent VTE in order to justify stopping anticoagulation if resultant death is the main parameter used for comparing the consequences of these competing outcomes. However, this body count approach clearly ignores each individual’s unique risk profile, preferences, and overall goals of care.
In my practice, I treat all patients with acute cancer-associated DVT with a minimum of 3 months of low-molecular-weight heparin; for those with PE, I treat for a minimum of 6 months because recurrent PE is more likely after index PE and is associated with significantly worse survival compared with recurrent DVT alone.10 After the initial 3 months and then every 3 months thereafter, I reassess the need and the patient’s tolerance of anticoagulation treatment. If the patient has metastatic disease or progressive cancer; requires ongoing chemotherapy or a thrombogenic regimen (eg, immunomodulatory drugs and dexamethasone for multiple myeloma or tamoxifen for breast cancer); has pancreatic, upper gastrointestinal (eg, esophageal, stomach, cholangiocarcinoma), lung, or ovarian cancer; or has glioblastoma, myeloproliferative neoplasm, or a previous history of VTE, then I recommend continuing anticoagulant treatment. I discuss the evidence available for low-molecular-weight heparins, warfarin, and direct oral anticoagulants and help the patient choose the anticoagulant treatment that best fits their needs and values. I do inform my patients that, currently, evidence for the use of direct oral anticoagulants for the first 3 to 6 months of treatment is limited to subgroup, retrospective analyses of highly selected patients with primarily early-stage cancer. I find that with proper education and support, the vast majority of patients will choose to do self-injection for up to 6 months (if cost is not an issue). Beyond 6 months, some will continue injections, and the remainder usually prefer a direct oral anticoagulant over warfarin to avoid laboratory testing. Before switching to an oral agent, I ensure there are no substantial drug interactions, liver and renal dysfunction, and diet or gastrointestinal concerns. At each visit, I again discuss anticoagulant options; review the signs and symptoms of recurrent DVT, PE, and intracranial hemorrhage; and remind the patient that urgent medical attention should be sought if these occurred. If the patient no longer has evidence of an active solid tumor or is in clinical remission of a hematological malignancy and is not receiving any systemic chemotherapy or is reaching the end of his or her cancer journey, then I usually recommend stopping anticoagulant therapy. After the initial 3 months of treatment, it is uncommon to have to stop anticoagulation for bleeding. For those with gastrointestinal or genitourinary cancers where bleeding is more common, I find that many patients will stop bleeding with a transient interruption of the anticoagulation treatment and appropriate intervention (eg, dose reduction, radiation, embolization). I do not recommend placement of a filter for recurrent VTE or as a substitute for anticoagulation treatment.
Summary
The use of secondary prophylaxis in patients with cancer-associated VTE requires thoughtful and thorough consideration at multiple time points in each patient’s unique cancer journey, with emphasis on providing anticoagulation when the risk of recurrent VTE is high and avoiding anticoagulant therapy when the risk is low. Real-world data show that poor adherence to guideline-recommended anticoagulation treatment appears to be associated with very high rates of recurrent thrombosis, bleeding, and mortality and that extensive education of both patients and physicians is needed.
Acknowledgments
The author thanks Erica Peterson and Cynthia Wu for their constructive comments.
Authorship
Contribution: A.Y.Y.L. reviewed the literature and wrote the manuscript.
Conflict-of-interest disclosure: A.Y.Y.L. has received research funding and honoraria from Bristol Myers Squibb and has served on the Board of Directors or an advisory committee, consulted, and received honoraria from Bayer, Pfizer, and LEO Pharma. Off-label drug use: None disclosed.
Correspondence: Agnes Y. Y. Lee, Division of Hematology, University of British Columbia and British Columbia Cancer Agency, 2775 Laurel St, 10th Floor, Vancouver, BC V5Z 1M9, Canada; e-mail: alee14@bccancer.bc.ca.
References
Author notes
This article was selected by the Blood and Hematology 2017 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2017. It is reprinted in Hematology Am Soc Hematol Educ Program. 2017;2017:128-135.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal