In this issue of Blood, Balsas et al define a SOX11-driven program supporting key cellular processes (cell adhesion, homing, and invasion) underlying some of the aggressive clinical and biological features of mantle cell lymphoma (MCL).1
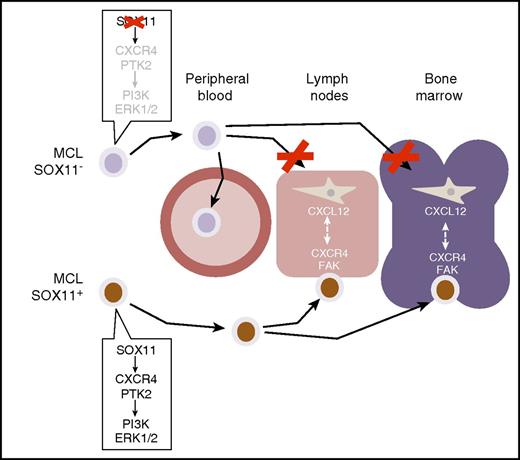
Balsas et al show how SOX11 expression in MCL (here marked with a brown nuclear staining) activates a gene expression program mediating cell adhesion and homing in lymph nodes and bone marrow. This gene program controls the expression of CXCR4 and FAK, which are critical mediators of cell-cell interactions driving the migration and homing of MCL cells into these tissues. SOX11 depletion, or lack of SOX11 expression as it occurs in a subset of indolent, leukemic non-nodal MCLs (blue-only cells), prevents these interactions, causing the redistribution of MCL cells to peripheral blood, away from homing tissue niches. PI3K, phosphatidylinositol 3-kinase. ERK, extracellular signal-regulated kinases.
Balsas et al show how SOX11 expression in MCL (here marked with a brown nuclear staining) activates a gene expression program mediating cell adhesion and homing in lymph nodes and bone marrow. This gene program controls the expression of CXCR4 and FAK, which are critical mediators of cell-cell interactions driving the migration and homing of MCL cells into these tissues. SOX11 depletion, or lack of SOX11 expression as it occurs in a subset of indolent, leukemic non-nodal MCLs (blue-only cells), prevents these interactions, causing the redistribution of MCL cells to peripheral blood, away from homing tissue niches. PI3K, phosphatidylinositol 3-kinase. ERK, extracellular signal-regulated kinases.
MCLs are lymphoid malignancies with aggressive clinical features and poor prognoses. Typically, MCLs are characterized by widespread dissemination and poor response to therapy, which may explain the disproportionate contribution of this rare lymphoma type to overall lymphoma mortality.2 In fact, the extension to extranodal sites (ie, gastrointestinal tract) and bone marrow (up to 60% of cases) implies a natural tendency for dissemination and homing to different tissue compartments, which in turn can affect cell survival and sensitivity to therapy.3 Previous studies had related this ability of MCL cells to home in different tissues to the expression of certain molecules involved in cell adhesion and cellular crosstalk, including CXCR4, VLA-4, CCL4, and TNFSF9,4 but the underlying mechanism remained largely unexplained.
Within the clinical spectrum of MCL, there is a small fraction of patient cases with indolent clinical behavior. These indolent MCL cases feature cyclin D1+ clonal B-cell populations with the hallmark t(11;14)(q13;q32) rearrangement found in a vast majority of MCLs but have a rather unusual clinical presentation, with prominent leukemic, non-nodal distribution and a nonprogressive clinical course that is in stark contrast to that of typical MCL.5-7 Most notably, they also lack expression of SOX11, a transcription factor that is overexpressed in MCL but not other B-cell non-Hodgkin lymphomas and is believed to play a prominent role in the biology of these malignancies.7,8 Reasonably, these observations prompted Balsas et al to investigate the possibility that SOX11 could also be responsible for the apparent differences in tissue dissemination between these 2 MCL groups.
The authors first used an integrative computational approach, revisiting previously published SOX11 chromatin immunoprecipitation microarray analysis and gene expression data,9 in combination with pathway analysis computational tools. Using this strategy, they found that cell migration and stromal stimulation signatures correlated with the expression of SOX11 and that CXCR4 and PTK2 (encoding for focal adhesion kinase [FAK]), 2 genes with known roles in these processes, were most prominent among all direct SOX11 gene targets (see figure). They further confirmed the latter observation in a series of MCL cell lines, where SOX11 expression was artificially manipulated through the constitutive expression of SOX11 or RNA interference with short hairpin RNAs. Interestingly, expression of the CXCR4 chemokine receptor had been previously reported to be elevated in a large fraction of MCL cell lines and had been shown to mediate cell migration and nurture crosstalk interactions between MCL cells and bone marrow stromal cells.4
Through a set of carefully crafted in vitro and in vivo experiments using engineered MCL cell lines and chemical inhibitors of CXCR4 and FAK activity, Balsas et al further confirmed and extended these fundamental observations. More importantly, they showed that expression of these programs depends (although not uniquely) on SOX11. Specifically, manipulation of SOX11 expression in xenografted MCL cell lines or, alternatively, chemical inhibition of CXCR4 and FAK signaling can alter the tissue distribution of the cancerous MCL cells. This results in contrasting patterns of widespread disease (bone marrow and lymph nodes) or disease significantly confined to peripheral blood, akin to that of patients with indolent MCL (see figure). These findings have obvious conceptual and translational implications, because they mechanistically explain how differential expression of SOX11 in the MCL disease spectrum may actually explain the exclusively leukemic, non-nodal presentation of SOX11− indolent MCL cases and their benign clinical behavior.
These results can also be translated into notable therapeutic considerations. Particularly, and as previously suggested by others,4 their findings may offer a foundation for a much-needed alternative approach to MCL treatment. Consistent with previous studies, Balsas et al showed that inhibition of the CXCR4/FAK axis prevented homing and crosstalk of MCL cells with peripheral tissue niches and that this effect resulted in increased sensitivity to conventional therapeutic agents like bortezomib by counteracting cell adhesion–mediated drug resistance. Cell adhesion–mediated drug resistance is a cytoprotective response supported by cell-cell interactions between cancerous and marrow stromal cells and has also been found in multiple myeloma and other hematologic malignancies that typically home in the bone marrow, like chronic myelogenous leukemia.10 Thus, in principle, blockade of MCL-stroma interactions with specific molecules would facilitate the mobilization of the cancerous cells into peripheral blood and away from protective tissue microenvironments, thereby increasing the therapeutic efficacy of conventional MCL therapies.
In summary, the study by Balsas et al provides an important and clarifying piece to the puzzle of MCL biology and further highlights SOX11 as a key driver of critical aspects of this disease. Furthermore, as occurs with all exciting science, it also raises some notable questions. Which other genes and programs are involved in the tissue homing and microenvironment interactions of MCL cells? To what extent are these programs controlled by SOX11? Are there any natural feed-forward signaling loops between lymphoid cells and the microenvironment that reinforce SOX11 expression? And if so, is the lack of SOX11 expression in indolent MCL cases a result of undiscovered defects in adhesion molecules or chemokine receptors? Finally, given the remarkable similarities between lymphoid and neuronal cells, one wonders if these programs may be reminiscent of those controlled by SOX11 during early neurogenesis and tissue remodeling. Overall, the findings of Balsas et al will keep many researchers busy with efforts that could further improve our understanding of MCL biology and could translate into unprecedented diagnostic and therapeutic strategies much needed for these patients.2
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal