Key Points
AL patients with an initial dFLC <50 mg/L represent a distinct clinical subgroup with mostly renal disease and a favorable prognosis.
These patients are evaluable for hematologic response including a novel low-dFLC partial response, which predict renal and overall survival.
Abstract
The difference between involved minus uninvolved serum free light chains (dFLC) has been established as an invaluable hematologic parameter in systemic amyloid light chain (AL) amyloidosis. However, patients with an initial dFLC level <50 mg/L are currently deemed not evaluable for response to therapy. Therefore, we aimed to characterize this subgroup of patients and to define novel hematologic response parameters. We retrospectively analyzed 783 AL patients newly diagnosed at our center between 2002 and 2016. Patients with a dFLC level <50 mg/L showed smaller bone marrow plasmacytosis compared to patients with a dFLC level ≥50 mg/L (7% vs 10%, P < .001), but no significant differences in all analyzed chromosomal aberrations. Cardiac involvement was less frequent (45% vs 80%, P < .001) and less severe (Mayo 2004 stage III: 18% vs 51%, P < .001), whereas kidney involvement was more prevalent (83% vs 53%, P < .001) and proteinuria was higher (7.3 g/L vs 5.0 g/L, P < .001). In multivariate analyses, a dFLC level <50 mg/L appeared to be an independent prognostic factor with respect to overall survival (hazard ratio [HR] = 0.50, P = .003) and renal survival (HR = 0.56, P = .020). Patients with a dFLC level <50 mg/L showed a higher proportion of complete hematologic response after first-line therapy compared to patients with a dFLC level ≥50 mg/L (39% vs 9%, P < .001). Receiver-operating characteristics analysis identified a low-dFLC partial response (dFLC <10 mg/L for patients with a dFLC between 20 and 50 mg/L), which predicted overall and renal survival already at 3 months after the start of therapy. Importantly, a parallel Italian study validated this new hematologic remission parameter. The outcome of prospective clinical trials might be adversely influenced by exclusion of the favorable clinical subgroup with an initial dFLC <50 mg/L. We propose the appreciation of dFLC in hematologic response assessment for all patients with a baseline dFLC >20 mg/L.
Introduction
Systemic amyloid light chain (AL) amyloidosis is a rare and life-threatening disease characterized by systemic deposition of misfolded free light chains (FLCs), which ultimately results in dysfunction of multiple organs, such as the heart, kidney, liver, or peripheral nervous system.1 The nephelometric FLC assay allows for a highly sensitive quantification of the involved free light chain (iFLC), representing to some extent the clonal size of the underlying B- or plasma cell disorder.2-5 The FLC test is beneficial in many respects. (1) It is effective in patients who lack an intact immunoglobulin, which is a frequent phenomenon in AL. (2) Even in patients with an intact immunoglobulin, the substantially shorter half-life of FLCs enables a more rapid detection of altered plasma cell activity compared with conventional methods like serum electrophoresis and quantification of the M-protein spike.6 (3) The high sensitivity of the FLC test is especially advantageous in cases of small plasma cell clones, which are frequently observed in AL amyloidosis.7 Because of all these advantages, the FLC test has evolved into an essential biomarker for the diagnosis and monitoring of treatment response and is a cornerstone of the international consensus guidelines for AL amyloidosis care.8
However, the unspecific accumulation of serum FLC in cases of impaired renal function might limit the applicability of iFLC blood levels,4 especially in older patients and AL patients with kidney failure. To account for this, the FLC κ/λ ratio is used for the diagnosis of the disease rather than absolute FLC levels alone, because the FLC ratio is not affected by age.4 Moreover, Dispenzieri et al9 introduced the difference between involved and uninvolved free light chain (dFLC) as a parameter for monitoring of the disease.
Several observations suggest that serum dFLC levels before treatment reflect disease severity: a higher serum dFLC level at initial presentation is associated with higher bone marrow plasmocytosis, poorer Karnofsky index score, and more severe heart involvement.3,10 Moreover, a 50% decrease in iFLC as well as dFLC levels on therapy is associated with significantly improved survival6,11 and is a more useful measure of hematologic response than the M-protein response.12
The current assessment of the hematologic response in AL amyloidosis is based on monitoring of the FLC serum levels (a >50% reduction of dFLC indicates a partial response [PR] and a decrease in dFLC to <40 mg/L indicates a very good PR) and immunofixation (IFE) (normalization of serum FLC levels, the κ/λ ratio, as well as negative IFE in serum and urine indicates a complete response).13
Although the detection limit of the FLC assay is <1 mg/L, the procedure is subject to several imprecisions.14,15 Due to such variability, a baseline dFLC of 50 mg/L is usually defined as not measurable for hematologic response.9,13 The respective patients account for up to 20%9 of AL cases and are excluded from most clinical trials because no hematologic response criterion besides complete hematologic response (aCR) can be applied to them.
The specific aims of this study were:
To comprehensively characterize AL patients with an initial dFLC <50 mg/L to estimate the consequences of their exclusion on the results of clinical trials; and
To evaluate hematologic response parameters for patients with an initial dFLC <50 mg/L to define an additional hematologic response criterion (low-dFLC PR) besides aCR, which was validated in an independent Italian cohort.
Methods
Patients
A total of 783 newly diagnosed patients with confirmed AL, who were treated and followed at the Heidelberg Amyloidosis Center between July 2002 and April 2016 and who obtained an FLC assay before initial treatment, were retrospectively studied for clinical parameters and cytogenetic aberrations. Cytogenetic analysis was performed after fluorescence in situ hybridization of CD138-enriched bone marrow aspirates, as previously outlined.16
Demographic and clinical information
All demographic and clinical information, including age, sex, and hematologic and organ-related laboratory tests were obtained from medical records. The study was approved by the Ethics Committee of the University of Heidelberg and was in accordance with the principles of the Helsinki declaration. All human participants gave written informed consent for retrospective analysis of clinical data.
Clinical staging, organ involvement, and hematologic and organ response assessment
Clinical staging, definition of organ involvement, as well as hematologic and organ response assessments were performed according to consensus standards13 and according to widely used definitions.8,17-20 General characteristics, including the cardiac stage (hereafter referred to as Mayo 200417,18 ) and renal stage,19 were evaluated in all patients. Overall survival was evaluated in all patients, whereas renal survival was only evaluated in patients with renal organ involvement. Renal response was only evaluated in patients with renal organ involvement who received anti–plasma cell therapy. Procedures are provided in detail in the supplemental Methods, available on the Blood Web site. The present study was performed in parallel with an Italian study (Milani et al21 ). Hematologic response criteria designed for patients with a low-dFLC burden were tested in the German cohort and validated in the Italian cohort.
Serum FLC concentrations
Serum FLC concentrations were obtained by using the Freelite κ and λ assays (Binding Site, Birmingham, UK). Analysis was performed on a Behring BN II nephelometer (Siemens Healthcare, Erlangen, Germany) and, after 29 May 2013, on a SPAPLUS System (Binding Site). The reference intervals for κ and λ FLC are 3.3 to 19.4 mg/L and 5.7 to 26.3 mg/L, respectively, and the κ/λ ratio diagnostic range is 0.26-1.65.
Statistical analysis
Statistical analysis was performed by using SPSS, version 22.0 statistical software (IBM, Armonk, NY) and statistical software environment R, version 3.3.2 together with the R packages OptimalCutpoints, version 1.1-3 and Survival, version 2.40-1. Continuous data were described with medians and ranges. The Wilcoxon rank-sum test was used to test for differences in continuous variables between groups. The Pearson's χ2 test or the Fisher’s exact test was used to test differences in nominal variables where applicable. Overall survival was defined as the time from the diagnosis to death or last contact. Renal survival was calculated as the time from the diagnosis to dialysis or last contact, whichever occurred first. Patients who underwent heart transplantation were censored at the day of transplantation for overall and renal survival analysis and excluded from response assessment. Overall and renal survival curves were constructed per Kaplan-Meier estimates, and comparisons were made by using the log-rank test. In Kaplan-Meier plots with >2 groups, the log-rank test was applied for pairwise comparisons of neighboring groups. Patients requiring dialysis before or at diagnosis were excluded from renal survival analysis (n = 14).
To investigate the impact of the criterion of dFLC <50 mg/L, we also performed multivariate Cox regression analyses with respect to overall and renal survival. In both analyses, the given therapy (none, bortezomib, melphalan and dexamethasone, high-dose melphalan [HDM], heart transplantation, or other) was a stratification factor. The additional covariates were age and renal stage for overall survival and age and Mayo 2004 stage for renal survival analyses.
The receiver operating characteristics (ROC) analysis was applied for all patients with a dFLC <50 mg/L to identify an optimal cut-off for the continuous variable dFLC after 3 months of follow-up, predicting the organ response at 12 months by using the Youden index criterion. The Youden index reaches the maximum for the optimal cut-off and is defined as YI(c) = max [Se(c) + Sp(c) – 1] for each dFLC value c. The area under the curve (AUC) lies between 0 and 1 and describes the performance of the classifier, where an AUC value of 0.5 is equivalent to a random guess.
All statistical tests were 2-sided. P < .05 was considered statistically significant.
Results
Clinical and cytogenetic characteristics
Patients with a dFLC <50 mg/L accounted for 106 (13.5%) of the 783 patients in our cohort. In comparison with patients with a dFLC ≥50 mg/L, there were no differences regarding age and sex (Table 1).
General characteristics and applied therapy
| Parameter . | All patients . | dFLC ≥50 mg/L . | dFLC<50 mg/L . | P† . |
|---|---|---|---|---|
| Patients, n | 783 | 677 | 106 | |
| General characteristics | ||||
| Sex, female | 312 (40) | 267 (39) | 45 (43) | .556 |
| Age at diagnosis, y | 62 (35-83) | 62 (35-83) | 62 (42-81) | .532 |
| Karnofsky index, % | 80 (40-100) | 80 (40-100) | 85 (40-100) | <.001 |
| Therapy | ||||
| None | 53 (7) | 43 (6) | 10 (9) | .001 |
| Bortezomib | 289 (37) | 262 (39) | 27 (26) | |
| Melphalan | 191 (24) | 167 (25) | 24 (23) | |
| HDM+ASCT | 149 (19) | 114 (17) | 35 (33) | |
| Other | 101 (13) | 91 (13) | 10 (9) | |
| Plasma cell–related factors | ||||
| dFLC, mg/L | 222 (1-12 078) | 273 (51-12 078) | 29 (1-49) | |
| dFLC >20 mg/L | 747 (95) | — | 70 (66) | — |
| Heavy chain in IFE | 328 (42) | 274 (41) | 54 (51) | .042 |
| Immunoglobulin m heavy chain, g/L | 10 (0.2-56.8) | 10.5 (0.2-56.8) | 7.8 (1.2-48.0) | .018 |
| M-protein spike, g/L | 8.6 (0.0-44.0) | 9.2 (0.0-44.0) | 6.6 (1.1-22.2) | .024 |
| Plasma cell count, % | 10 (1-90) | 10 (1-90) | 7 (1-80) | <.001 |
| iFLC = λ | 625 (80) | 534 (79) | 91 (86) | .096 |
| Organ involvement | ||||
| ANS | 83 (11) | 72 (11) | 11 (10) | .936 |
| GIT | 276 (35) | 249 (37) | 27 (26) | .023 |
| Heart | 586 (75) | 538 (80) | 48 (45) | <.001 |
| Kidney | 449 (57) | 361 (53) | 88 (83) | <.001 |
| Liver | 163 (21) | 143 (21) | 20 (19) | .595 |
| Lung | 16 (2) | 14 (2) | 2 (2) | .899 |
| PNS | 148 (19) | 130 (19) | 18 (17) | .587 |
| Soft tissue | 317 (41) | 290 (43) | 27 (26) | .001 |
| Organs, n | 3 (2-3) | 3 (2-3) | 2 (1-3) | .001 |
| Cardiac parameters | ||||
| NT-proBNP, ng/L | 3223 (22-565 442) | 3923 (22-565 442) | 395 (22-150 806) | <.001 |
| Mayo 2004 I | 125 (17) | 86 (14) | 39 (44) | <.001 |
| Mayo 2004 II | 257 (36) | 223 (36) | 34 (38) | |
| Mayo 2004 III | 335 (47) | 319 (51) | 16 (18) | |
| Renal parameters | ||||
| eGFR, mL/min | 65.0 (1.9-262.4) | 63.4 (1.9-262.4) | 71.6 (7.5-149.1) | .212 |
| eGFR <30 mL/min | 107 (14) | 94 (14) | 13 (12) | .605 |
| eGFR <15 mL/min | 32 (4) | 27 (4) | 5 (5) | .753 |
| Dialysis at diagnosis | 15 (2) | 14 (2) | 1 (1) | .432 |
| Albuminuria, mg/d | 1186 (1-17 160) | 537 (1-15 167) | 4751 (3-17 160) | <.001 |
| Proteinuria, g/d | 2.1 (0.0-22.3) | 1.5 (0.0-20.5) | 6.0 (0.0-22.3) | <.001 |
| Renal stage* | ||||
| I | 123 (29) | 104 (30) | 19 (22) | .291 |
| II | 215 (50) | 169 (49) | 46 (54) | |
| III | 90 (21) | 69 (20) | 21 (24) |
| Parameter . | All patients . | dFLC ≥50 mg/L . | dFLC<50 mg/L . | P† . |
|---|---|---|---|---|
| Patients, n | 783 | 677 | 106 | |
| General characteristics | ||||
| Sex, female | 312 (40) | 267 (39) | 45 (43) | .556 |
| Age at diagnosis, y | 62 (35-83) | 62 (35-83) | 62 (42-81) | .532 |
| Karnofsky index, % | 80 (40-100) | 80 (40-100) | 85 (40-100) | <.001 |
| Therapy | ||||
| None | 53 (7) | 43 (6) | 10 (9) | .001 |
| Bortezomib | 289 (37) | 262 (39) | 27 (26) | |
| Melphalan | 191 (24) | 167 (25) | 24 (23) | |
| HDM+ASCT | 149 (19) | 114 (17) | 35 (33) | |
| Other | 101 (13) | 91 (13) | 10 (9) | |
| Plasma cell–related factors | ||||
| dFLC, mg/L | 222 (1-12 078) | 273 (51-12 078) | 29 (1-49) | |
| dFLC >20 mg/L | 747 (95) | — | 70 (66) | — |
| Heavy chain in IFE | 328 (42) | 274 (41) | 54 (51) | .042 |
| Immunoglobulin m heavy chain, g/L | 10 (0.2-56.8) | 10.5 (0.2-56.8) | 7.8 (1.2-48.0) | .018 |
| M-protein spike, g/L | 8.6 (0.0-44.0) | 9.2 (0.0-44.0) | 6.6 (1.1-22.2) | .024 |
| Plasma cell count, % | 10 (1-90) | 10 (1-90) | 7 (1-80) | <.001 |
| iFLC = λ | 625 (80) | 534 (79) | 91 (86) | .096 |
| Organ involvement | ||||
| ANS | 83 (11) | 72 (11) | 11 (10) | .936 |
| GIT | 276 (35) | 249 (37) | 27 (26) | .023 |
| Heart | 586 (75) | 538 (80) | 48 (45) | <.001 |
| Kidney | 449 (57) | 361 (53) | 88 (83) | <.001 |
| Liver | 163 (21) | 143 (21) | 20 (19) | .595 |
| Lung | 16 (2) | 14 (2) | 2 (2) | .899 |
| PNS | 148 (19) | 130 (19) | 18 (17) | .587 |
| Soft tissue | 317 (41) | 290 (43) | 27 (26) | .001 |
| Organs, n | 3 (2-3) | 3 (2-3) | 2 (1-3) | .001 |
| Cardiac parameters | ||||
| NT-proBNP, ng/L | 3223 (22-565 442) | 3923 (22-565 442) | 395 (22-150 806) | <.001 |
| Mayo 2004 I | 125 (17) | 86 (14) | 39 (44) | <.001 |
| Mayo 2004 II | 257 (36) | 223 (36) | 34 (38) | |
| Mayo 2004 III | 335 (47) | 319 (51) | 16 (18) | |
| Renal parameters | ||||
| eGFR, mL/min | 65.0 (1.9-262.4) | 63.4 (1.9-262.4) | 71.6 (7.5-149.1) | .212 |
| eGFR <30 mL/min | 107 (14) | 94 (14) | 13 (12) | .605 |
| eGFR <15 mL/min | 32 (4) | 27 (4) | 5 (5) | .753 |
| Dialysis at diagnosis | 15 (2) | 14 (2) | 1 (1) | .432 |
| Albuminuria, mg/d | 1186 (1-17 160) | 537 (1-15 167) | 4751 (3-17 160) | <.001 |
| Proteinuria, g/d | 2.1 (0.0-22.3) | 1.5 (0.0-20.5) | 6.0 (0.0-22.3) | <.001 |
| Renal stage* | ||||
| I | 123 (29) | 104 (30) | 19 (22) | .291 |
| II | 215 (50) | 169 (49) | 46 (54) | |
| III | 90 (21) | 69 (20) | 21 (24) |
Categorical data are shown as counts (percentages of respective total); continuous data are shown as medians (ranges).
ANS, autonomic nervous system; Bortezomib, bortezomib-based regimens; eGFR, estimated glomerular filtration rate; GIT, gastrointestinal tract; HDM+ASCT, HDM followed by autologous stem cell transplantation; Melphalan, melphalan-dexamethasone; Mayo 2004, score based on Dispenzieri et al18 ; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PNS, peripheral nervous system; Renal stage, based on Palladini et al.19
Renal stage applied only to patients with kidney involvement.
P values in italics indicate statistical significance.
Patients with a dFLC <50 mg/L showed significantly lower bone marrow plasma cell counts, M-protein spikes, and concentrations of the respective monoclonal heavy chain compared to patients with dFLC ≥50 mg/L, whereas the mere presence of a monoclonal heavy chain in IFE was more frequent in these patients (Table 1).
Frequencies of cytogenetic aberrations were comparable to other data already reported by our group.16 There were no significant differences between patients with a dFLC <50 mg/L and ≥50 mg/L (supplemental Table 1) both regarding single chromosomes or cytogenetic risk groups. The most prominent nonsignificant differences were lower frequencies of t(11;14) (52% vs 60%, P = .156), del8p21 (3% vs 7%, P = .177), del17p13 (1% vs 4%, P = .272), and gain5p15 (8% vs 14%, P = .192).
As expected, the heart and kidneys were the most frequently involved organs (Table 1). Compared with patients with dFLC ≥50 mg/L, less organs were involved in patients with a dFLC <50 mg/L. These patients also showed significantly less soft tissue involvement. Most importantly, heart involvement was significantly less frequent and less severe (Mayo 2004 score) in patients with a dFLC <50 mg/L. Consequently, patients with a dFLC <50 mg/L had a significantly better performance status as quantified by the Karnofsky index, and high-dose chemotherapy followed by autologous stem cell transplantation was offered more frequently to these patients. Intriguingly, kidney involvement was significantly more common in patients with an initial dFLC <50 mg/L, and proteinuria was significantly higher. In contrast, there was a trend to a better renal function at diagnosis in patients with an initial dFLC <50 mg/L. Consequently, renal stage distribution was not significantly different between the 2 groups (Table 1).
Overall and renal survival
Patients with an initial dFLC <50 mg/L showed a significantly better median overall survival regardless of treatment type (bortezomib, melphalan, or HDM + autologous stem cell transplantation; Figure 1). Of note, this advantage persisted when only patients with heart involvement were included in the analysis (Figure 2A). However, the risk of renal failure did not differ between the dFLC ≥/<50 mg/L subgroups (Figure 3A), or when all patients, irrespective of kidney involvement, were analyzed (supplemental Figure 2). As expected, the Mayo 2004 score predicted overall survival (Figure 2B-D), and the renal stage19 predicted renal survival (Figure 3B-D) in patients with renal involvement. Importantly, this was also the case in the subgroups of patients with a dFLC ≥50 mg/L and a dFLC <50 mg/L.
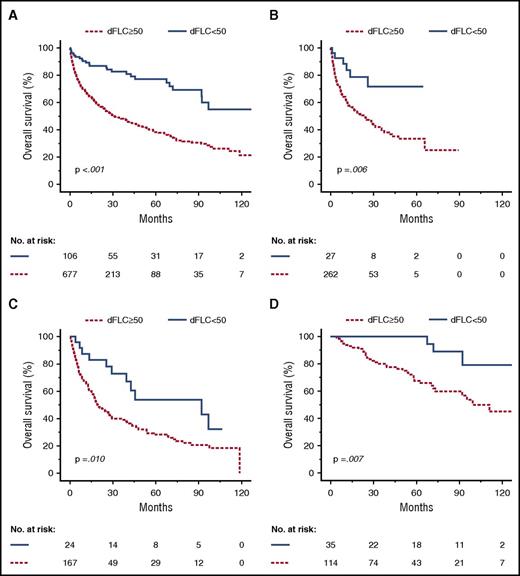
Overall survival according to group (dFLC <50 or ≥50 mg/L) and first-line treatment. Kaplan-Meyer plots depict overall survival. (A) Entire cohort. Median overall survival: 31.2 months (dFLC ≥50 mg/ml) vs not reached (dFLC <50 mg/L). (B) Bortezomib-based chemotherapy. Median overall survival: 23.0 months (dFLC ≥50 mg/L) vs not reached (dFLC <50 mg/L). (C) Melphalan-dexamethasone. Median overall survival: 19.6 months (dFLC ≥50 mg/L) vs 92.0 months (dFLC <50 mg/L). (D) HDM followed by autologous stem cell transplantation. Median overall survival: 99.3 months (dFLC ≥50 mg/L) vs not reached (dFLC <50 mg/L).
Overall survival according to group (dFLC <50 or ≥50 mg/L) and first-line treatment. Kaplan-Meyer plots depict overall survival. (A) Entire cohort. Median overall survival: 31.2 months (dFLC ≥50 mg/ml) vs not reached (dFLC <50 mg/L). (B) Bortezomib-based chemotherapy. Median overall survival: 23.0 months (dFLC ≥50 mg/L) vs not reached (dFLC <50 mg/L). (C) Melphalan-dexamethasone. Median overall survival: 19.6 months (dFLC ≥50 mg/L) vs 92.0 months (dFLC <50 mg/L). (D) HDM followed by autologous stem cell transplantation. Median overall survival: 99.3 months (dFLC ≥50 mg/L) vs not reached (dFLC <50 mg/L).
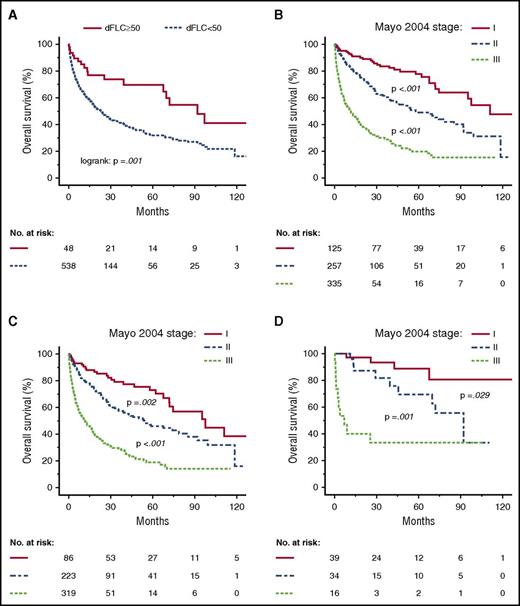
Overall survival according to group (dFLC <50 or ≥50 mg/L) and heart involvement. (A) Overall survival of patients with amyloid heart involvement. Median overall survival: 23.0 months (dFLC ≥50 mg/L) vs 92.0 months (dFLC <50 mg/L). (B) Overall survival of all patients according to Mayo 2004 stages. Median overall survival: 110.9 months (I) vs 58.0 months (II) vs 11.5 months (III). (C) Overall survival of patients with an initial dFLC ≥50 mg/L according to Mayo 2004 stages. Median overall survival: 97.5 months (I) vs 54.2 months (II) vs 11.7 months (III). (D) Overall survival of patients with an initial dFLC <50 mg/L according to Mayo 2004 stages. Median overall survival: not reached (I) vs 92.0 months (II) vs 6.9 months (III).
Overall survival according to group (dFLC <50 or ≥50 mg/L) and heart involvement. (A) Overall survival of patients with amyloid heart involvement. Median overall survival: 23.0 months (dFLC ≥50 mg/L) vs 92.0 months (dFLC <50 mg/L). (B) Overall survival of all patients according to Mayo 2004 stages. Median overall survival: 110.9 months (I) vs 58.0 months (II) vs 11.5 months (III). (C) Overall survival of patients with an initial dFLC ≥50 mg/L according to Mayo 2004 stages. Median overall survival: 97.5 months (I) vs 54.2 months (II) vs 11.7 months (III). (D) Overall survival of patients with an initial dFLC <50 mg/L according to Mayo 2004 stages. Median overall survival: not reached (I) vs 92.0 months (II) vs 6.9 months (III).
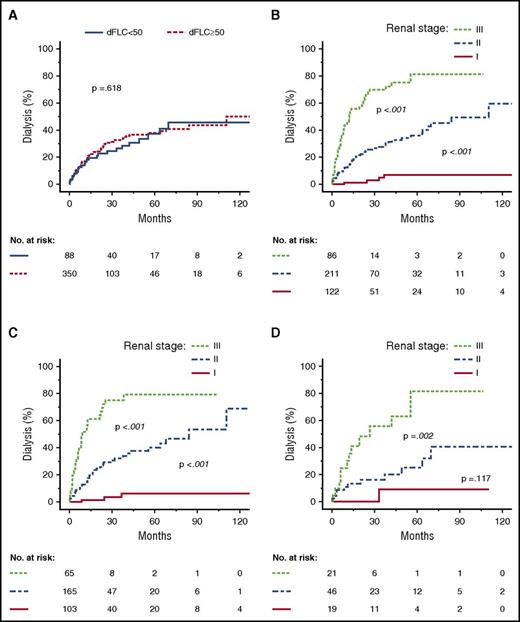
Progression to dialysis within 36 months according to group (dFLC <50 or ≥50 mg/L) and renal stage. Kaplan-Meyer plots depict renal survival. (A) All patients with renal involvement. (B) All patients with renal involvement, stratified by renal stage. (C) Patients with renal involvement and an initial dFLC ≥50 mg/L, stratified by renal stage. (D) Patients with renal involvement and an initial dFLC <50 mg/L, stratified by renal stage.
Progression to dialysis within 36 months according to group (dFLC <50 or ≥50 mg/L) and renal stage. Kaplan-Meyer plots depict renal survival. (A) All patients with renal involvement. (B) All patients with renal involvement, stratified by renal stage. (C) Patients with renal involvement and an initial dFLC ≥50 mg/L, stratified by renal stage. (D) Patients with renal involvement and an initial dFLC <50 mg/L, stratified by renal stage.
We also performed multivariate Cox regression analyses of the dFLC <50 mg/L criterion with respect to overall survival (including the age and Mayo 2004 stage covariates, stratified by therapy) and renal survival (including the age and renal stage covariates, stratified by therapy). These multivariate analyses suggest a dFLC <50 mg/L as an additional independent prognostic factor for both overall survival and renal survival with a hazard ratio (HR) of 0.5 (95% confidence interval [CI]: 0.32-0.72) and a HR of 0.56 (95% CI: 0.35-0.91), respectively (Table 2).
DFLC <50 mg/L shows prognostic value with respect to overall and renal survival in multivariate Cox regression analyses, including age, Mayo 2004 stage, and renal stage
| Criterion . | Overall survival (n = 717 with 358 events) . | Renal survival (n = 420 with 111 events) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P* . | HR (95% CI) . | P* . | |
| Age (1-y increase) | 1.01 (0.99-1.02) | .340 | 1.02 (1.00-1.05) | .060 |
| dFLC <50 mg/L (yes vs no) | 0.50 (0.32-0.79) | .003 | 0.56 (0.35-0.91) | .020 |
| Mayo stage II vs I | 1.73 (1.14-2.62) | .010 | Not included | |
| Mayo stage III vs I | 3.71 (2.46-5.61) | <.001 | Not included | |
| Renal stage II vs I | Not included | 10.15 (3.67-28.10) | <.001 | |
| Renal stage III vs I | Not included | 38.09 (13.50-107.47) | <.001 | |
| Criterion . | Overall survival (n = 717 with 358 events) . | Renal survival (n = 420 with 111 events) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P* . | HR (95% CI) . | P* . | |
| Age (1-y increase) | 1.01 (0.99-1.02) | .340 | 1.02 (1.00-1.05) | .060 |
| dFLC <50 mg/L (yes vs no) | 0.50 (0.32-0.79) | .003 | 0.56 (0.35-0.91) | .020 |
| Mayo stage II vs I | 1.73 (1.14-2.62) | .010 | Not included | |
| Mayo stage III vs I | 3.71 (2.46-5.61) | <.001 | Not included | |
| Renal stage II vs I | Not included | 10.15 (3.67-28.10) | <.001 | |
| Renal stage III vs I | Not included | 38.09 (13.50-107.47) | <.001 | |
For multivariate analysis of overall survival, all patients were analyzed. For multivariate analysis of renal survival, only patients with kidney involvement were included. Both analyses were stratified by therapy. Covariates were age, dFLC <50 mg/L, Mayo 2004 stage (only overall survival), and renal stage (only renal survival). HRs are shown with corresponding 95% CIs.
P values in italics indicate statistical significance.
Response assessment
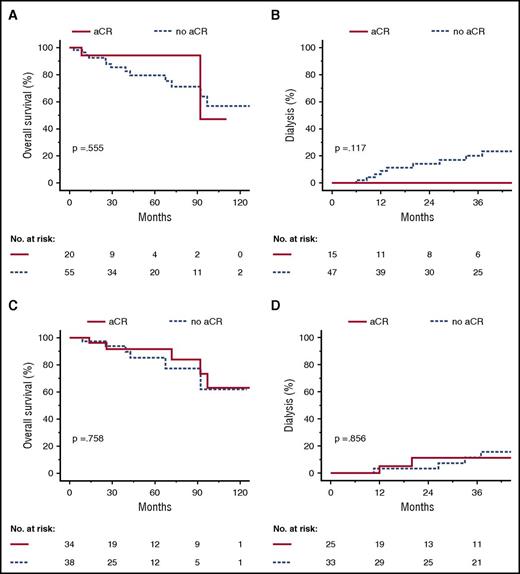
Patients with an initial dFLC <50 mg/L had higher aCR rates compared with patients with a dFLC ≥50 mg/L (Table 3). This difference was independent of the treatment regimen (supplemental Tables 2 and 3). To estimate the value of aCR in patients with an initial dFLC <50 mg/L, we performed a landmark analysis based on hematologic response at 3 and 6 months of follow-up (Figure 4). There was no significant difference between responders and nonresponders with respect to overall survival. However, none of the patients with aCR at 3 months required dialysis within the following 36 months, whereas 9 of the nonresponders did, but this difference did not reach statistical significance in our cohort.
Hematologic and renal response
| . | dFLC ≥50 mg/L . | dFLC <50 mg/L . | P* . |
|---|---|---|---|
| Hematologic response | |||
| 3 mo | n = 565 | n = 85 | |
| aCR | 34 (6) | 20 (24) | <.001 |
| Low-dFLC PR | n.a. | 18 (21) | n.a. |
| VGPR | 137 (24 | n.a. | n.a. |
| PR | 132 (23) | n.a. | n.a. |
| No response | 144 (26) | 41 (48) | .001 |
| Early death | 118 (21) | 6 (7) | .002 |
| 6 mo | n = 554 | n = 88 | |
| aCR | 48 (9) | 34 (39) | <.001 |
| Low-dFLC PR | n.a. | 14 (16) | n.a. |
| VGPR | 130 (23) | n.a. | n.a. |
| PR | 112 (20) | n.a. | n.a. |
| No response | 77 (14) | 31 (35) | .001 |
| Early death | 187 (34) | 9 (10) | <.001 |
| Renal response | |||
| 6 mo | n = 476 | n = 85 | |
| Response | 130 (27) | 22 (26) | Eval.: .004 |
| Stable | 61 (13) | 29 (34) | ITT: <.001 |
| Progression | 98 (21) | 25 (29) | |
| Early death | 187 (39) | 9 (11) | |
| 12 mo | n = 501 | n = 81 | |
| Response | 122 (24) | 25 (31) | Eval.: .123 |
| Stable | 34 (7) | 15 (19) | ITT: <.001 |
| Progression | 105 (21) | 29 (36) | |
| Early death | 240 (48) | 12 (15) |
| . | dFLC ≥50 mg/L . | dFLC <50 mg/L . | P* . |
|---|---|---|---|
| Hematologic response | |||
| 3 mo | n = 565 | n = 85 | |
| aCR | 34 (6) | 20 (24) | <.001 |
| Low-dFLC PR | n.a. | 18 (21) | n.a. |
| VGPR | 137 (24 | n.a. | n.a. |
| PR | 132 (23) | n.a. | n.a. |
| No response | 144 (26) | 41 (48) | .001 |
| Early death | 118 (21) | 6 (7) | .002 |
| 6 mo | n = 554 | n = 88 | |
| aCR | 48 (9) | 34 (39) | <.001 |
| Low-dFLC PR | n.a. | 14 (16) | n.a. |
| VGPR | 130 (23) | n.a. | n.a. |
| PR | 112 (20) | n.a. | n.a. |
| No response | 77 (14) | 31 (35) | .001 |
| Early death | 187 (34) | 9 (10) | <.001 |
| Renal response | |||
| 6 mo | n = 476 | n = 85 | |
| Response | 130 (27) | 22 (26) | Eval.: .004 |
| Stable | 61 (13) | 29 (34) | ITT: <.001 |
| Progression | 98 (21) | 25 (29) | |
| Early death | 187 (39) | 9 (11) | |
| 12 mo | n = 501 | n = 81 | |
| Response | 122 (24) | 25 (31) | Eval.: .123 |
| Stable | 34 (7) | 15 (19) | ITT: <.001 |
| Progression | 105 (21) | 29 (36) | |
| Early death | 240 (48) | 12 (15) |
Data are shown as counts (percentages of n). See “Methods” for definition of aCR and PR. Organ response criteria were applied according to Gertz et al and Palladini et al.8,19 Dialysis was assigned as renal progression.
Early death, death before envisaged follow-up; Eval., P value with respect to evaluable patients; ITT, intention-to-treat P value includes patients who experienced early death; Low-dFLC PR, drop of dFLC <10 mg/L if the initial dFLC was 0.20 mg/L (only in cases without aCR and for patients with a dFLC <50 mg/L); n, number of patients evaluable for hematologic or organ response, including early death; na, not applicable; No response, less than PR, progress, or start of second-line chemotherapy; VGPR, very good PR (only patients with a dFLC ≥50 mg/L).
P values in italics indicate statistical significance.
Overall survival and renal survival according to complete response at 3 and 6 months in patients with an initial dFLC <50 mg/L. Landmark analysis at 3 months after diagnosis (A-B) and 6 months after diagnosis (C-D). Kaplan-Meyer plots depict overall survival (A,C) and progression to dialysis (B,D).
Overall survival and renal survival according to complete response at 3 and 6 months in patients with an initial dFLC <50 mg/L. Landmark analysis at 3 months after diagnosis (A-B) and 6 months after diagnosis (C-D). Kaplan-Meyer plots depict overall survival (A,C) and progression to dialysis (B,D).
We evaluated organ response as defined by Palladini et al.19 Because kidney involvement was predominant in patients with an initial dFLC <50 mg/L, we focused on renal organ response (Table 3). The fraction of patients with renal response was not different between the dFLC ≥/<50 mg/L subgroups. However, the group of patients with an initial dFLC <50 mg/L had a higher fraction of nonresponders or patients with stable organ parameters, whereas the group of patients with an initial dFLC ≥50 mg/L had a higher fraction of patients who experienced early death. Of note, the initial proteinuria and complete hematologic remission rates were both higher in the dFLC <50 mg/L subgroup.
New definition of hematologic response in patients with a dFLC <50 mg/L
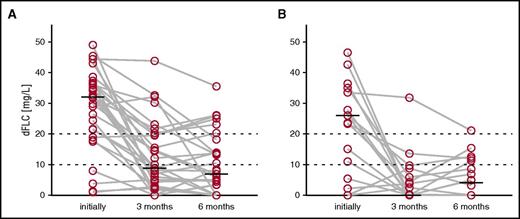
Next, we looked into organ and hematologic responders in the dFLC <50 mg/L subgroup. Out of 19 evaluable patients with kidney organ response at 12 months of follow-up, only 4 patients (21%) had achieved an aCR at 3 months. This fraction was not higher than that of patients without renal organ response (11 patients, 28%; P = .559, χ2 test). We then compared the median dFLC levels of patients with and without organ response at 12 months of follow-up (Figure 5). Patients with renal organ response at 12 months showed significantly lower dFLC levels at 3 months (P = .001). The dFLC levels were not significantly different at 6 months of follow-up (P = .198). Moreover, median dFLC levels at 3 months landmark (P = .005) and at 6 months landmark (P = .009) were significantly higher if patients required dialysis within 36 months. This suggests that in patients with a dFLC <50 mg/L as well, a reduction in the dFLC level within 3 months might translate into favorable renal organ response and renal survival.
dFLC levels at different time points of patients with and without renal organ response at 12 months. Each gray line represents 1 case. (A) Patients without renal organ response at 12 months of follow-up (n = 18). (B) Patients with renal organ response at 12 months of follow-up (n = 29).
dFLC levels at different time points of patients with and without renal organ response at 12 months. Each gray line represents 1 case. (A) Patients without renal organ response at 12 months of follow-up (n = 18). (B) Patients with renal organ response at 12 months of follow-up (n = 29).
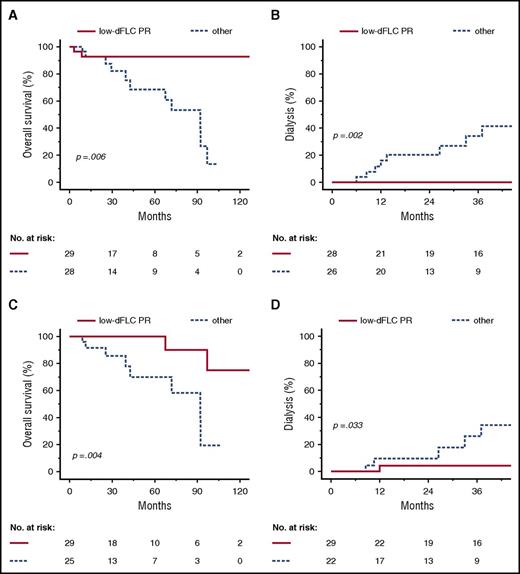
To identify an optimal cut-off for the dFLC level at 3 months of follow-up, we performed a ROC analysis predicting organ response at 12 months, which yielded a value of 9.81 mg/L (Youden criterion; area under the ROC curve = 0.697; group distribution was 20 responders vs 52 nonresponders). Based on this analysis and analogous to a very good PR of patients with an initial dFLC >50 mg/L, we assumed a dFLC threshold of 10 mg/L in patients whose baseline dFLC was >20 mg/L to be clinically practical and meaningful (low-dFLC PR). Out of 106 patients with an initial dFLC <50 mg/L, 100 patients (94%) were evaluable for aCR, 70 patients (66%) had an initial dFLC ≥20 mg/L and were thus evaluable for the proposed low-dFLC PR, and 103 patients (97%) were either evaluable for aCR or low-dFLC PR. No difference in overall survival was seen in patients who had a baseline dFLC <20 mg/L compared with those with a dFLC of 20-49 mg/L (median survival not reached vs 97 months, P = .471). Compared with the use of aCR alone, we identified 18 additional hematologic responders at 3 and 6 months of follow-up. Importantly, in a landmark analysis from 3 and 6 months of follow-up, low-dFLC PR predicted median overall survival (Figure 6A,C; not reached vs 92 months for both time points, P = .006 and P = .004, log-rank test) as well as renal survival (Figure 6B,D; patients requiring dialysis at 36 months after landmark 0% vs 41% and 4% vs 34%, P = .002 and P = .033, log-rank test). Finally and most importantly, the proposed low-dFLC PR was validated in a parallel Italian study (Milani et al21 ). Table 4 summarizes the proposed hematologic response parameters with the associated renal response and survival rates in patients with an initial dFLC <50 mg/L.
Overall survival and renal survival according to low-dFLC PR at 3 and 6 months in patients with an initial dFLC <50 mg/L. Landmark analysis at 3 months after diagnosis (A-B) and 6 months after diagnosis (C-D). Kaplan-Meyer plots depict overall survival (A,C) and progression to dialysis (B,D).
Overall survival and renal survival according to low-dFLC PR at 3 and 6 months in patients with an initial dFLC <50 mg/L. Landmark analysis at 3 months after diagnosis (A-B) and 6 months after diagnosis (C-D). Kaplan-Meyer plots depict overall survival (A,C) and progression to dialysis (B,D).
Proposed hematologic response parameters with associated renal response and survival rates in patients with an initial dFLC <50 mg/L
| Hematologic response at 3 mo . | n (%) . | Renal response at 12 mo . | Overall survival at 63 mo, % . | Renal survival at 39 mo, % . |
|---|---|---|---|---|
| aCR | 20 (27) | 4 (21) | 94 | 100 |
| Low-dFLC PR | 29 (51) | 11 (85) | 93 | 100 |
| No response | 41 (39) | 8 (38) | 73 | 67 |
| Hematologic response at 3 mo . | n (%) . | Renal response at 12 mo . | Overall survival at 63 mo, % . | Renal survival at 39 mo, % . |
|---|---|---|---|---|
| aCR | 20 (27) | 4 (21) | 94 | 100 |
| Low-dFLC PR | 29 (51) | 11 (85) | 93 | 100 |
| No response | 41 (39) | 8 (38) | 73 | 67 |
Data are shown as counts (percentages of evaluable n). See “Methods” for definition of aCR. Organ response criteria were applied according to Gertz et al and Palladini et al.8,19
Low-dFLC PR, drop of dFLC to <10 mg/L if the initial dFLC was >20 mg/L (also counted in patients with aCR); No response, no aCR or low–dFLC PR, progress, start of second-line chemotherapy, or death.
Discussion
Our first aim was to comprehensively characterize AL patients with an initial dFLC <50 mg/L to estimate the possible consequences of their systematic exclusion on the results of the clinical trials. We demonstrate that patients with low amyloidogenic FLC levels at first diagnosis show remarkable clinical features, including a favorable prognosis, presumably due to less severe heart involvement and a greater hematologic response (greater fraction of patients with complete remission) after first-line chemotherapy. Published data of aCR rates for AL patients treated with HDM range from 31% to 39% with conventional therapies and from 12% to 42% with new drugs.1 Our response data for all patients is within this range. However, patients with an initial dFLC <50 mg/L account for ∼50% of aCR cases in our cohort, resulting in excellent hematologic response rates (up to 57% aCR rate at 6 months of follow-up after HDM, intention-to-treat analysis). Exclusion of these patients in our cohort resulted in an inferior hematologic response compared with reported data of other groups (4%-8% aCR after conventional therapies up to only 15% aCR after HDM). It should be considered that the choice of therapy in patients with AL amyloidosis is influenced by the severity of organ involvement as well as the magnitude of the plasma cell clone. Therefore, comparison of different treatment strategies in a nonrandomized fashion might lead to the wrong conclusions. We showed that patients with an initial dFLC <50 mg/L have a favorable prognosis even after stratification for different treatment strategies, including conventional and high-dose chemotherapy. Therefore, systematic exclusion of patients with an initial dFLC <50 mg/L from clinical trials could cause substantial bias with a negative influence on overall survival and hematologic response.
We also showed that the clinical subgroup of patients with an initial dFLC <50 mg/L is characterized by a distinct pattern of organ involvement (predominance of kidney involvement, whereas the heart and soft tissue are less involved) and a small plasma cell clone (bone marrow plasma cell count, median M-protein spike, and median concentration of monoclonal heavy chain). A unique phenotype of light chains with high glomerular toxicity or a higher susceptibility of the glomerulus in the respective patients could explain these observations. We did not find a significant association of a dFLC level <50 mg/L with genetic aberrations on single chromosomes or cytogenetic risk groups (high risk or hyperdiploidy).
The clinical experience with AL amyloidosis patients suggests that the inherent propensity of amyloidogenic FLC to misfold and deposit is different in each patient and for each organ. Moreover, it was shown that high baseline dFLC levels (>180 mg/L) predict early organ recovery in patients who have a complete hematologic response.11,22 The authors assumed that the amyloidogenic threshold could be relatively greater in patients with initial high FLC levels. In contrast, an analysis of prediagnostic sera from 20 AL cases also suggest a heterogeneity in the degree of proliferation in the underlying plasma cell clone as a possible reason for high or low amyloidogenic FLC levels at first diagnosis.23 It is likely that both the different dynamics of the underlying plasma cell disorder as well as the unique biology of amyloidogenic FLCs or their fragments contribute to the wide clinical spectrum of AL patients.
In a second step, we evaluated hematologic response parameters for patients with an initial dFLC level <50 mg/L to define an additional hematologic response criterion (low-dFLC PR). So far, patients with a dFLC <50 mg/L are only evaluable for aCR. However, achievement of aCR at 3 or 6 months of follow-up was not associated with significant improvement of renal and overall survival in our patient cohort. Nevertheless, a parallel Italian study (Milani et al21 ) showed a prognostic value for aCR at 6 months with respect to both renal and overall survival. In our view, this apparent discrepancy shows the potential differences in the sensitivity of IFE electrophoreses between centers, which makes comparison of aCR in patients with a small plasma cell clone less reliable. This is also an additional argument for the application of the FLC test in response assessment of patients with a small plasma cell clone.
According to the current definitions, >75% of our patients with a dFLC <50 mg/L did not respond to chemotherapy within 3 months. This contrasts with the 75% of patients with renal organ response that did not achieve an aCR and suggests that IFE and FLC ratio alone are not sufficient to monitor meaningful hematologic responses in patients with a dFLC <50 mg/L. However, patients with renal organ response at 12 months showed significantly lower levels of serum dFLC at 3 months of follow-up, whereas patients who required dialysis within 36 months posttherapy had higher serum dFLC levels at 3 and 6 months. Therefore, we hypothesized that the FLCs in serum are valuable in monitoring hematologic response in patients with an initial dFLC <50 mg/L as well. ROC analysis based on organ response at 12 months found the optimal dFLC cut-off for 3 months of follow-up to be 9.81 mg/L. Based on this analysis and analogous to the very good PR of patients with an initial dFLC >50 mg/L, we assumed a dFLC threshold of 10 mg/L to be clinically practical and meaningful. To account for imprecisions in the FLC test, we assumed that only patients with a baseline dFLC of >20 mg/L should be evaluated for this response criterion (low-dFLC PR). Seventy (66%) of our 106 patients with a dFLC <50 mg/L had an initial dFLC >20 mg/L and would therefore be evaluable for this response criterion.
The proposed low-dFLC PR was validated in the Italian cohort (Milani et al21 ). Both of our groups showed significantly superior overall and renal survival rates for hematologic responders at 3 and 6 months of follow-up. Our data suggest that dFLC monitoring in patients with an initial dFLC between 20 and 50 mg/L might be most informative at 3 months of follow-up: dFLC levels at 3 months of follow-up are significantly different between organ responders and nonresponders, whereas there is no statistically significant difference at 6 months of follow-up. This is in accordance with the apparent deterioration of the prognostic value of CR after 6 months compared with 3 months in our cohort and suggests that an early and deep response is necessary to translate into a meaningful advantage in outcome, especially in patients with low initial amyloidogenic FLC levels.
The main limitations of our study are the retrospective design and the rather small subgroup of patients with an initial dFLC <50 mg/L. However, Milani et al show a similar characterization of the patients with a dFLC <50 mg/L. The fact that our response findings are “working” in 2 independent patient populations, despite relatively small numbers, further corroborates the discriminating ability of the proposed criterion.
To our knowledge, this is the first study to show that an FLC response in patients with a dFLC <50 mg/L is associated with meaningful organ response. Our proposed definition of hematologic response identifies patients with PR to first line chemotherapy who were previously classified as nonresponders. This could allow for better monitoring of the plasma cell disease in this subgroup of patients, which accounts for up to 20% of AL amyloidosis patients, and facilitates the inclusion of these patients with proper stratification into prospective clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Rita Ziehl, medical data manager, for assistance in the collection of clinical data.
Authorship
Contribution: T.D., U.H., and S.O.S. contributed to study conception and design; T.D., A.J., N.B., U.H., and S.O.S. contributed to acquisition, analysis, and interpretation of data; and T.D., T.B., C.K., N.B., A.J., H.G., A.D.H., U.H., and S.O.S. wrote the manuscript or revised it critically for important intellectual content.
Conflict-of-interest disclosure: H.G. discloses research support from Celgene, Janssen, Chugai, Novartis, BMS, serves on the advisory boards for Janssen, Celgene, Novartis, Amgen Takeda, BMS, and has received honoraria from Janssen. U.H. has received travel grants from Janssen and Pfizer, serves on the advisory boards for Prothena and Pfizer, and has received honoraria from Janssen. S.O.S. has received a travel grant from Binding Site, research support from Celgene, Janssen, Sanofi, serves on the advisory boards for Janssen and Prothena, and has received honoraria from Celgene, Janssen, and Millenium. The remaining authors declare no competing financial interests.
Correspondence: Ute Hegenbart, Amyloidosis Center, Heidelberg University Hospital, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: ute.hegenbart@med.uni-heidelberg.de; and Stefan O. Schönland, Amyloidosis Center, Heidelberg University Hospital, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: stefan.schoenland@med.uni-heidelberg.de.
References
Author notes
U.H. and S.O.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal