Abstract
Antiplatelet therapy is of proven benefit in coronary artery disease and a number of other clinical settings. This article reviews platelet function, molecular targets of antiplatelet agents, and clinical indications for antiplatelet therapy before focusing on a frequent question to hematologists about the 2 most commonly used antiplatelet therapies: Could the patient be aspirin “resistant” or clopidogrel “resistant”? If so, should results of a platelet function test be used to guide the dose or type of antiplatelet therapy? Whether such guided therapy is of clinical benefit to patients has been a source of controversy. The present article reviews this subject in the context of 2 prototypical clinical cases. Available evidence does not support the use of laboratory tests to guide the dose of aspirin or clopidogrel in patients with so-called aspirin or clopidogrel “resistance.”
Introduction
Platelets are small cells of great importance in thrombosis.1 Platelets have a critical role in coronary artery disease (the leading cause of death in all countries except those in the lowest economic stratum) and in other common diseases, including stroke and peripheral artery disease. Antiplatelet therapy is therefore of proven benefit in these clinical settings (Table 1).
Available antiplatelet therapies
| Mechanism . | Agent . | Structure . | Route/dosing . | Clinical use . |
|---|---|---|---|---|
| COX-1 inhibition | Aspirin | Acetylsalicylic acid | Oral/daily | Coronary artery disease, cerebrovascular disease, PAD, primary prevention, stents, CABG, CEA |
| Irreversible P2Y12 antagonism | Ticlopidine | Thienopyridine | Oral/twice daily | Cerebrovascular disease, coronary stents (now rarely used) |
| Clopidogrel | Thienopyridine | Oral/daily | Prior myocardial infarction, ischemic stroke, or symptomatic PAD, as monotherapy; ACS or coronary stenting, as part of dual antiplatelet therapy with aspirin | |
| Prasugrel | Thienopyridine | Oral/daily | Patients with ACS treated with stents, as part of dual antiplatelet therapy with aspirin | |
| Reversible P2Y12 antagonism | Ticagrelor | Cyclopentyl-triazolo-pyrimidine | Oral/twice daily | Patients with ACS, as part of dual antiplatelet therapy with aspirin |
| Cangrelor | ATP analog | IV | PCI when not pretreated with oral P2Y12 antagonist | |
| GPIIb-IIIa inhibition | Abciximab | Murine human chimeric Fab fragment | IV | PCI |
| Eptifibatide | KGD-containing cyclic heptapeptide | IV | ACS, PCI | |
| Tirofiban | Nonpeptide mimetic based on RGD | IV | ACS, PCI | |
| PAR1 inhibition | Vorapaxar | Tricyclic himbacine derivative | Oral/daily | Prior myocardial infarction, PAD |
| PDE Inhibition | Cilostazol | 2-Oxoquinoline derivative | Oral/twice daily | PAD |
| Dipyridamole | Pyrimidopyridine derivative | Oral/twice daily | Stroke or TIA when used with aspirin | |
| Combination | Aspirin/dipyridamole (Aggrenox) | Acetylsalicylic acid/pyrimidopyridine derivative | Oral/twice daily | Stroke, TIA |
| Mechanism . | Agent . | Structure . | Route/dosing . | Clinical use . |
|---|---|---|---|---|
| COX-1 inhibition | Aspirin | Acetylsalicylic acid | Oral/daily | Coronary artery disease, cerebrovascular disease, PAD, primary prevention, stents, CABG, CEA |
| Irreversible P2Y12 antagonism | Ticlopidine | Thienopyridine | Oral/twice daily | Cerebrovascular disease, coronary stents (now rarely used) |
| Clopidogrel | Thienopyridine | Oral/daily | Prior myocardial infarction, ischemic stroke, or symptomatic PAD, as monotherapy; ACS or coronary stenting, as part of dual antiplatelet therapy with aspirin | |
| Prasugrel | Thienopyridine | Oral/daily | Patients with ACS treated with stents, as part of dual antiplatelet therapy with aspirin | |
| Reversible P2Y12 antagonism | Ticagrelor | Cyclopentyl-triazolo-pyrimidine | Oral/twice daily | Patients with ACS, as part of dual antiplatelet therapy with aspirin |
| Cangrelor | ATP analog | IV | PCI when not pretreated with oral P2Y12 antagonist | |
| GPIIb-IIIa inhibition | Abciximab | Murine human chimeric Fab fragment | IV | PCI |
| Eptifibatide | KGD-containing cyclic heptapeptide | IV | ACS, PCI | |
| Tirofiban | Nonpeptide mimetic based on RGD | IV | ACS, PCI | |
| PAR1 inhibition | Vorapaxar | Tricyclic himbacine derivative | Oral/daily | Prior myocardial infarction, PAD |
| PDE Inhibition | Cilostazol | 2-Oxoquinoline derivative | Oral/twice daily | PAD |
| Dipyridamole | Pyrimidopyridine derivative | Oral/twice daily | Stroke or TIA when used with aspirin | |
| Combination | Aspirin/dipyridamole (Aggrenox) | Acetylsalicylic acid/pyrimidopyridine derivative | Oral/twice daily | Stroke, TIA |
CABG, coronary artery bypass graft; CEA, carotid endarterectomy; KGD, Lys-Gly-Asp; PAD, peripheral artery disease; PDE, phosphodiesterase; RGD, Arg-Gly-Asp; TIA, transient ischemic attack.
Frequent questions to hematologists about aspirin and clopidogrel, the 2 most commonly used antiplatelet therapies, are: Could the patient be aspirin “resistant” or clopidogrel “resistant”? If so, should results of a platelet function test be used to guide the dose or type of antiplatelet therapy? Whether such guided therapy is of clinical benefit to patients has been a source of controversy. The present article reviews this subject in the context of 2 prototypical clinical cases.
Platelet function and molecular targets of antiplatelet agents
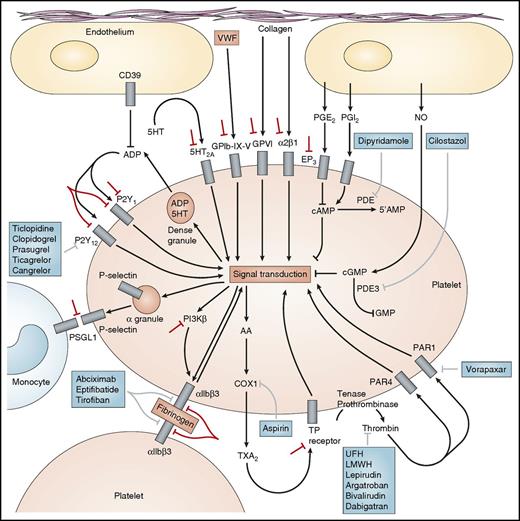
Platelets normally circulate in a resting discoid form maintained via inhibition of platelet activation by (1) endothelial-derived nitric oxide, (2) endothelial-derived prostaglandin I2 (prostacyclin), and (3) scavenging of adenosine diphosphate (ADP) by endothelial surface CD39 (Figure 1).2 Initial platelet adhesion to damaged vessel walls is mediated by exposed collagen binding to platelet surface glycoprotein (GP) VI and integrin α2β1 and by von Willebrand factor (VWF) binding to the platelet surface GPIb-X-V complex (Figure 1). Thrombin, generated by the coagulation cascade, is a potent activator of human platelets via 2 platelet surface receptors: protease-activated receptor (PAR) 1 and PAR4 (Figure 1). Three groups of platelet surface receptors provide important positive feedback loops for platelet activation: (1) P2Y1 and P2Y12 receptors are stimulated by ADP released from platelet dense granules; (2) 5-HT2A receptors are stimulated by serotonin released from platelet dense granules; and (3) the thromboxane prostanoid receptor is stimulated by thromboxane (TX) A2 generated by the platelet cyclooxygenase (COX) 1-dependent signaling pathway (Figure 1). Platelet-to-platelet aggregation is mediated by fibrinogen and, at high shear flow, VWF binding to the activated molecular conformation of integrin αIIbβ3 (GPIIb-IIIa) (Figure 1). Platelet-monocyte adhesion is initially mediated by the binding of platelet surface P-selectin (which is expressed only on the platelet surface after platelet degranulation) to its constitutively expressed counterreceptor, P-selectin glycoprotein ligand 1, on the monocyte surface (Figure 1).
Molecular targets of antiplatelet agents. US Food and Drug Administration–approved antiplatelet agents are shown in blue boxes. Novel antiplatelet agents in development are shown by red bars. 5-HT, 5-hydroxytryptamine (serotonin); LMWH, low-molecular-weight heparin; NO, nitric oxide; PG, prostaglandin; PI3Kβ, β isoform of phosphoinositide 3-kinase; PSGL-1, P-selectin glycoprotein ligand 1; UFH, unfractionated heparin. Modified with permission from Michelson AD. Nat Rev Drug Discovery. 2010;9:154-169.
Molecular targets of antiplatelet agents. US Food and Drug Administration–approved antiplatelet agents are shown in blue boxes. Novel antiplatelet agents in development are shown by red bars. 5-HT, 5-hydroxytryptamine (serotonin); LMWH, low-molecular-weight heparin; NO, nitric oxide; PG, prostaglandin; PI3Kβ, β isoform of phosphoinositide 3-kinase; PSGL-1, P-selectin glycoprotein ligand 1; UFH, unfractionated heparin. Modified with permission from Michelson AD. Nat Rev Drug Discovery. 2010;9:154-169.
The molecular targets of the US Food and Drug Administration–approved antiplatelet agents are shown in Table 1 and Figure 1 (blue boxes).2 Aspirin acetylates serine 529 on COX1 and thereby blocks TXA2 generation (Figure 1). P2Y12 antagonists (ticlopidine, clopidogrel, prasugrel, ticagrelor, and cangrelor) prevent ADP-dependent amplification of stable platelet aggregation and secretion (Figure 1). GPIIb-IIIa (αIIbβ3) antagonists (abciximab, eptifibatide, and tirofiban) block the binding of fibrinogen and, at high shear, VWF to GPIIb-IIIa on adjacent platelets and thereby block platelet-to-platelet aggregation (Figure 1). Vorapaxar is a PAR1 antagonist (Figure 1). Dipyridamole and cilostazol are phosphodiesterase inhibitors that have both antiplatelet (Figure 1) and vasodilatory effects. Unfractionated heparin, low-molecular-weight heparin, and direct thrombin inhibitors (lepirudin, argatroban, bivalirudin, and dabigatran), unlike PAR1 antagonists, are anticoagulants rather than specific antiplatelet drugs. However, their inhibition of thrombin results in less platelet activation (Figure 1) (although some studies show that unfractionated heparin can activate platelets).
Indications for antiplatelet therapy
Antiplatelet therapy has a number of evidence-based indications (Table 1). Aspirin should be given at the time of a suspected acute myocardial infarction. Once the myocardial infarction or acute coronary syndrome (ACS) is confirmed by abnormal cardiac biomarkers and/or ischemic electrocardiographic changes, a second oral antiplatelet agent such as clopidogrel or ticagrelor can be considered.3-5 If a coronary stent is placed, the second antiplatelet agent is mandatory, and in the ACS patient this can be clopidogrel, prasugrel, or ticagrelor.3,4,6 This dual antiplatelet therapy (DAPT) should be continued for at least a year unless major bleeding issues arise. Beyond the first year after an ACS, options include aspirin monotherapy or clopidogrel monotherapy.7,8 In patients at low bleeding risk and high ischemic risk, continued DAPT with aspirin plus clopidogrel 75 mg or aspirin plus ticagrelor 60 mg twice daily can be considered.9-11
For patients who undergo elective coronary stenting for stable ischemic heart disease, current guidelines in both the United States and Europe recommend 6 months of DAPT if a second-generation drug-eluting stent is used.12,13 Studies are ongoing to determine if even shorter durations of DAPT followed by antiplatelet monotherapy would be sufficient. For bare metal stents, which are used much less frequently nowadays, 1 month of DAPT is the minimum sufficient period per the guidelines, although existing data do not actually support different durations for bare metal stents vs second-generation drug-eluting stents.14
For patients with stable angina treated medically or who have had coronary artery bypass surgery, aspirin is recommended. Aspirin is also used in patients with symptomatic peripheral artery disease, although the evidence suggests that clopidogrel is likely more efficacious.8 Ticagrelor is not superior to clopidogrel in patients with peripheral artery disease.15 Cilostazol is used in patients with peripheral artery disease, although more for treating intermittent claudication than as an antiplatelet drug per se. The literature does not support antiplatelet therapy for asymptomatic, incidentally discovered peripheral artery disease. Patients with a history of ischemic stroke or transient ischemic attack are also often treated with aspirin; clopidogrel is also an option in these patients.7,8 The combination of clopidogrel plus aspirin is not recommended for cerebrovascular disease per se, unless there is another independent indication for DAPT. Dipyridamole plus aspirin is another option in patients with a history of ischemic stroke or transient ischemic attack.16
For primary prevention (that is, patients who have no clinical signs suggesting atherosclerosis), the use of aspirin is common but controversial. Several trials are ongoing to determine if high-risk patients, such as those with diabetes mellitus, truly benefit from aspirin therapy in the contemporary era. The uncertainty is based on whether the small expected reduction in ischemic events (and other possible but unproven beneficial effects of aspirin [eg, primary prevention of venous thromboembolism, primary prevention of colorectal cancers17 ]) is offset by the bleeding risks.
Vorapaxar, a relatively new oral antiplatelet agent, is approved in the United States for secondary prevention in patients with prior myocardial infarction or peripheral artery disease.18 In Europe, it is approved in patients with a history of myocardial infarction. To date, use of this drug has been limited.
Intravenous antiplatelet therapy has also been extensively studied. Although the oral GPIIb-IIIa inhibitors did not have a favorable risk/benefit profile,19 the intravenous GPIIb-IIIa inhibitors (abciximab, eptifibatide, and tirofiban) were demonstrated to reduce ischemic events in patients undergoing percutaneous coronary intervention (PCI), especially those with ACS.20 Eptifibatide and tirofiban were additionally shown to reduce ischemic events in ACS patients when started as upstream therapy before cardiac catheterization and continued through PCI or until coronary artery bypass surgery.
More recently, the intravenous P2Y12 receptor antagonist, cangrelor, has been introduced into clinical practice.21-24 Cangrelor has been studied across the spectrum of patients undergoing PCI who have not been adequately pretreated with oral P2Y12 receptor antagonists or intravenous GPIIb-IIIa inhibitors. Cangrelor was found to reduce periprocedural ischemic events significantly, with no significant excess in the number of blood transfusions per patient compared with a strategy of clopidogrel loading in the catheterization laboratory after the coronary anatomy is delineated. Thus, in hospitals in which prompt catheterization of ACS patients occurs or in which there is no pretreatment with oral P2Y12 receptor antagonists before elective stenting, cangrelor is a very useful option. Another potential use of cangrelor is in bridging patients who are perceived to need transitioning from oral P2Y12 receptor antagonists before surgical procedures but who are still in the early period after coronary stenting.25 However, this is an unapproved use of cangrelor and the dose is much lower than the dose used during PCI.
Case 1
You are asked by cardiology to do a hematology consult on a 65-year-old man with a history of acute myocardial infarction. He takes aspirin 81 mg daily for secondary prevention of thrombosis. Clinical tests are available to determine whether he is “aspirin resistant.” The consult question is: would this patient benefit clinically by using 1 of these tests to determine whether his dose of aspirin should be modified?
What are the clinically available tests for monitoring aspirin therapy?
Because the mechanism of aspirin’s antiplatelet effect is blockade of TXA2 generation (Figure 1), measurement of TXB2, the stable metabolite of TXA2, is the most optimal and direct way to measure the platelet inhibitory effect of aspirin.26 Two methods are available: serum TXB2 and urinary 11-dehydro TXB2. An alternative method for the determination of aspirin’s antiplatelet effect is to stimulate platelets with arachidonic acid (Figure 1) and use 1 of a number of readouts (eg, light transmission aggregometry in platelet-rich plasma, impedance platelet aggregometry in whole blood [eg, Multiplate], VerifyNow Aspirin Test [a whole blood, point-of-care method that includes fibrinogen-coated beads], or the thromboelastogram [TEG] PlateletMapping system).26 Major limitations of all these methods include the lack of pathological shear and the absence of the endothelium. Although not yet clinically available, a recently described microfluidic method resolves these problems by including a chemically fixed endothelial cell lining that retains its ability to modulate hemostasis under continuous pathophysiological shear rates, even after a few days of storage.27 The platelet function analyzer (PFA)-100 method includes shear but is not recommended because it is less specific for aspirin’s effect.26 An additional major limitation of all methods for monitoring the antiplatelet effects of aspirin is that the results of different methods correlate poorly with each other and with the gold standard serum TXB2 method.28,29
Is increasing the dose of aspirin above 75-100 mg beneficial?
The generally accepted aspirin dose is 75, 81, or 100 mg, depending on the available commercial formulations of the drug in different countries.17 Because 75 mg daily is at least twice as high as the lowest dose necessary and sufficient to fully inhibit platelet COX-1 activity, there are no significant differences in the antiplatelet effects of doses ranging between 75 and 100 mg.17 Randomized comparisons of higher vs lower aspirin doses in patients with ACS30 and cerebrovascular disease31,32 showed no evidence of superiority of higher vs lower doses of aspirin.17 Although aspirin 325 mg can be used as a loading dose in the setting of ACS or acute ischemic stroke, prescribing aspirin 325 mg daily for long-term treatment does not produce any additional benefit, while exposing the patient to unnecessary possible side effects: gastrointestinal damage, bleeding complications, and possibly negative interactions with ticagrelor.4,17 Nevertheless, the 2014 American Heart Association/American College of Cardiology non-ST elevation acute coronary syndromes guidelines still allow an aspirin maintenance dose range of 81 to 325 mg per day, with some degree of preference given to 81 mg daily.33 Although aspirin is usually administered daily, there is evidence that twice-daily dosing may be beneficial in patients with diabetes mellitus, obesity, essential thrombocythemia, myeloproliferative neoplasms, and coronary artery bypass surgery, possibly because of increased platelet turnover.17,34-37
What accounts for the reported phenomenon of aspirin resistance?
Aspirin resistance is a widely used term in the medical literature38 and there are reports of associations between lack of response to aspirin-dependent tests and major adverse cardiovascular effects.39 However, if “resistance” is defined in pharmacological terms as the failure of aspirin intake to fully inactivate its target, platelet COX-1, as evidenced by lack of inhibition of thromboxane B2 generation, then aspirin resistance is either a very rare phenomenon40-42 or does not exist.17 Based on this pharmacologic definition, a study of 400 healthy volunteers failed to identify a single case of aspirin resistance.40 “Pseudo-resistance,” reflecting delayed and reduced drug absorption and therefore impairment of the pharmacodynamic effect, may complicate enteric-coated, but not immediate-release, aspirin administration.17,40,43,44 Patient nonadherence and/or drug-drug interactions between aspirin and another nonsteroidal anti-inflammatory drug (NSAID, eg, ibuprofen,45 naproxen46-48 ) is probably responsible for many literature reports of aspirin resistance. 17,49 Both noncompliance and drug-drug interactions can be assessed by serum TXB2 measurements before and 24 hours after a witnessed aspirin intake.17,42 Increasing the dose of aspirin will not improve platelet inhibition if the patient is not taking aspirin or is on a concomitant NSAID medication.17 However, improving the patient’s motivation, stopping NSAID therapy, or switching to an NSAID that does not interfere with the antiplatelet action of aspirin (eg, celecoxib, diclofenac)45,50 will likely increase the extent and duration of platelet inhibition by a standard low-dose aspirin regimen.17 The effect of ibuprofen can be bypassed by giving subjects aspirin 2 hours before a single daily dose of ibuprofen.45 However, of more clinical relevance, the inhibitory effects of daily low-dose aspirin on platelets are competitively inhibited by the prolonged use of multiple daily doses of ibuprofen, even when the aspirin is administered before a dose of the NSAID.45 Doubling the dose of aspirin administered once daily will not prolong the duration of its antiplatelet effect in diabetic or thrombocythemic patients with their faster recovery of platelet COX-1 during the 24-hour dosing interval, whereas increasing the dosing frequency to twice a day will result in persistent suppression of COX-1 activity throughout the dosing interval.17,34-37
Summary of case 1
There is no evidence that this 65-year-old man with a history of acute myocardial infarction would benefit clinically by using a test to determine whether his dose of aspirin should be modified. However, there are a number of strategies available to optimize his low-dose aspirin therapy, as summarized in Table 2.
Optimizing low-dose aspirin therapy
| Suggested action . | Reference . | Clinical implication . |
|---|---|---|
| Use the lowest effective dose (ie, 75-100 mg daily) | 30,-32 | Maximize clinical efficacy; minimize gastrointestinal toxicity and drug-drug interactions |
| Consider BID dosing in patients with type 2 diabetes mellitus and essential thrombocythemia | 34,,-37 | Ensure persistent inhibition of platelet function throughout the dosing interval; clinical benefit remains untested |
| Prefer nonenteric-coated formulations | 40,43,44 | Improve extent and duration of platelet inhibition |
| Improve adherence | 42 | Avoid misclassification of “resistance” |
| Avoid concomitant administration of ibuprofen and naproxen | 45,,-48,50 | Avoid interference with the antiplatelet effect of low-dose aspirin |
| Avoid concomitant administration of gastrotoxic medications (NSAIDs and high-dose corticosteroids) | 89 | Improve gastrointestinal safety |
| Consider proton pump inhibitors in high-risk patients. Consider eradication in Helicobacter pylori–positive patients. | 63,90 | Improve gastrointestinal safety |
| Suggested action . | Reference . | Clinical implication . |
|---|---|---|
| Use the lowest effective dose (ie, 75-100 mg daily) | 30,-32 | Maximize clinical efficacy; minimize gastrointestinal toxicity and drug-drug interactions |
| Consider BID dosing in patients with type 2 diabetes mellitus and essential thrombocythemia | 34,,-37 | Ensure persistent inhibition of platelet function throughout the dosing interval; clinical benefit remains untested |
| Prefer nonenteric-coated formulations | 40,43,44 | Improve extent and duration of platelet inhibition |
| Improve adherence | 42 | Avoid misclassification of “resistance” |
| Avoid concomitant administration of ibuprofen and naproxen | 45,,-48,50 | Avoid interference with the antiplatelet effect of low-dose aspirin |
| Avoid concomitant administration of gastrotoxic medications (NSAIDs and high-dose corticosteroids) | 89 | Improve gastrointestinal safety |
| Consider proton pump inhibitors in high-risk patients. Consider eradication in Helicobacter pylori–positive patients. | 63,90 | Improve gastrointestinal safety |
Modified with permission from Patrono.17
Case 2
You are asked by cardiology to do a hematology consult on a 55-year-old man who recently had a coronary artery stent placed during a PCI. In addition to aspirin 81 mg daily, he takes clopidogrel 75 mg daily for secondary prevention of thrombosis. Clinical tests are available to determine whether he is “clopidogrel resistant.” The consult question is: would this patient benefit clinically by using 1 of these tests to determine whether his dose of clopidogrel should be modified or whether a different antiplatelet agent should be substituted?
What are the clinically available tests for monitoring clopidogrel therapy?
Because the mechanism of clopidogrel’s antiplatelet effect is blockade of the P2Y12 ADP receptor (Figure 1), clopidogrel’s effect on platelet function can be measured by stimulation of platelets with ADP and the use of 1 of a number of readouts (eg, phosphorylation of vasodilator-stimulated phosphoprotein measured by flow cytometry or enzyme-linked immunosorbent assay, light transmission aggregometry in platelet-rich plasma, impedance platelet aggregometry in whole blood [eg, Multiplate], VerifyNow PRUTest assay, the TEG PlateletMapping system, or the INNOVANCE PFA-200 System).26 As for aspirin monitoring, limitations of these methods include the lack of pathological shear (except in the INNOVANCE PFA-200 System) and the absence of the endothelium. As described previously, a recently described method resolves these problems27 but is not yet clinically available. As for aspirin monitoring,28,29 an additional major limitation of all methods for monitoring the antiplatelet effects of clopidogrel is that the results of different methods correlate poorly with each other.51,52 Furthermore, measurements of platelet reactivity vary over time in a significant proportion of patients.53 Thus, treatment adjustment according to platelet function testing at a single time point might not be sufficient for guiding antiplatelet therapy. In contrast, monitoring the effect of clopidogrel therapy by CYP2C19 genotyping (as discussed in the following sections) does not vary over time.54-56
What accounts for the reported phenomenon of clopidogrel resistance?
Antiplatelet therapy with clopidogrel reduces coronary thrombotic events in patients with ACS.2 However, platelet inhibition by clopidogrel is highly variable, and patients with reduced platelet inhibition (commonly referred to as clopidogrel resistance, but probably better termed high on-treatment platelet reactivity57 ) have an increased risk for major adverse cardiovascular events.57,58 Esterases degrade ∼85% of absorbed clopidogrel, leaving only ∼15% to be converted by the cytochrome P-450 family of enzymes to the active metabolite required for inhibition of the platelet ADP receptor P2Y12.51 Variability in clopidogrel pharmacokinetics and pharmacodynamics has been attributed to a number of factors, including patient noncompliance, absorption (eg, diet or polymorphisms in the transporter molecule ABCB154,59,60 ), smoking (which alters cytochrome P-450 levels),61,62 polymorphisms in CYP2C19,54-56 drug-drug interactions (eg, proton pump inhibitors63-65 ), intrinsic variation in platelet function before exposure to clopidogrel,66-68 and other clinical factors (obesity, renal dysfunction, diabetes mellitus, age, reduced left ventricular function, inflammation).58 However, other as-yet unidentified factors also contribute to high on-treatment platelet reactivity.51 A 600-mg loading dose (as opposed to a 300-mg loading dose) of clopidogrel overcomes some of these issues.69
What is the evidence that guided therapy based on a test of clopidogrel resistance is clinically beneficial in patients receiving clopidogrel?
Platelet function tests and thrombosis
Increased risk for cardiac ischemic events during short- and long-term follow-up in clopidogrel-treated patients with high on-treatment platelet reactivity has been identified by a number of ADP-dependent platelet function assays, including light transmission aggregometry, VerifyNow, Multiplate, TEG PlateletMapping system, INNOVANCE PFA-200 System. and vasodilator-stimulated phosphoprotein.57,58,70 The development of user-friendly point-of-care methods (eg, VerifyNow) to assess platelet reactivity to ADP has increased the frequency of platelet function testing in clinical practice.71 Indeed, large observational studies have established an independent relationship between high on-treatment platelet reactivity as measured by point-of-care platelet function testing and ischemic events in patients undergoing PCI.71 However, the relationship between high on-treatment platelet reactivity and ischemic events is not robust in patients with ACS managed with medical therapy.72 Some small studies had suggested that guided therapy based on the degree of inhibition of platelet function tests by clopidogrel may be beneficial in reducing ischemic events.73 These data resulted in low-level recommendations for use of platelet function monitoring tests in clinical guidelines from the American Heart Association, American College of Cardiology, and European Society of Cardiology.71 However, 3 subsequent large prospective randomized controlled trials, Gauging Responsiveness With A VerifyNow Assay-Impact On Thrombosis And Safety (GRAVITAS) (2800 patients),74 The Assessment by a Double Randomization of a Conventional Antiplatelet Strategy versus a Monitoring-guided Strategy for Drug-Eluting Stent Implantation and of Treatment Interruption versus Continuation One Year after Stenting (ARCTIC) (2440 patients),75 and Assessment of a Normal Versus Tailored Dose of Prasugrel After Stenting in Patients Aged > 75 Years to Reduce the Composite of Bleeding, Stent Thrombosis and Ischemic Complications (ANTARCTIC) (877 patients),76 failed to demonstrate that personalized antiplatelet therapy based on point-of-care assessment of platelet function is effective in reducing ischemic events. Although there were limitations to each of these 3 major trials (Table 3), the results do not provide support for the concept of changing antiplatelet therapy based on the results of platelet function monitoring tests. Thus, high on-treatment platelet reactivity may be a nonmodifiable clinical risk factor in clopidogrel-treated patients.
Comparison of 3 major randomized controlled trials measuring the utility of platelet function tests for guiding antiplatelet therapy in patients with high on-treatment platelet reactivity (clopidogrel “resistance”)
| Trial feature . | GRAVITAS74 . | ARCTIC75 . | ANTARCTIC76 . |
|---|---|---|---|
| Patient characteristics | PCI with DES: −58% stable CAD, –27% unstable angina without MI, −15% ACS | PCI with DES: 27% NSTE-ACS | Patients aged ≥75 years undergoing PCI with stenting for ACS |
| Number of patients | 2800 | 2440 | 877 |
| Platelet function test | VerifyNow P2Y12 assay HPR cutoff: ≥230 PRU | VerifyNow P2Y12 assay HPR cutoff: ≥235 PRU or ≤15% inhibition | VerifyNow P2Y12 assay HPR cutoff: ≥208 PRU, LPR cutoff: ≤85 PRU |
| Treatment type/dose in the monitoring group | Clopidogrel 600-mg loading dose, followed by 150-mg maintenance dose | Clopidogrel 600-mg loading dose plus clopidogrel 150 mg daily (−90%) or prasugrel 10 mg daily (−10%) | Prasugrel 5 mg daily (55%); prasugrel 10 mg (4%); clopidogrel 75 mg (39%) |
| Efficacy outcome (monitoring group vs conventional-treatment group) | 6-month cardiovascular death, nonfatal MI, or stent thrombosis: 2.3% vs 2.3%; HR 1.01, P = .97 | 1-year death, MI, stent thrombosis, stroke, or urgent revascularization: 34.6% vs 31.1%; HR 1.13, P = 0.10 | 1-year cardiovascular death, MI, stroke, stent thrombosis, urgent revascularization, and BARC-defined bleeding (types 2, 3, or 5): 28% vs 28%; HR 1.0, P = .98 |
| Safety outcome | Severe or moderate GUSTO bleeding: 1.4% vs 2.3%; HR = 0.59, P = .10 | Major STEEPLE bleeding:2.3% vs 3.3%; HR = 0.57, P = .12 | BARC-defined bleeding (types 2, 3, or 5): 21% vs 20%; HR 1.04, P = .77 |
| Limitations | • Low-risk patient cohort resulted in low event rate, hence underpowered to test the utility of PFM | • Low-risk patient cohort | • Uniform strategy for first 14 days: utility of PFM not tested in early period |
| • Suboptimal remedy to overcome HPR | • Suboptimal remedy to overcome H PR in majority of patients | • Therapeutic approach in the PFM group is primarily to reduce bleeding | |
| • Randomization done 12 or 24 hours after PCI, missing periprocedural events | • Underpowered for postdischarge event occurrence | • Approximately 8.7% of the PFM group did not undergo PFM on either day 14 or day 28 |
| Trial feature . | GRAVITAS74 . | ARCTIC75 . | ANTARCTIC76 . |
|---|---|---|---|
| Patient characteristics | PCI with DES: −58% stable CAD, –27% unstable angina without MI, −15% ACS | PCI with DES: 27% NSTE-ACS | Patients aged ≥75 years undergoing PCI with stenting for ACS |
| Number of patients | 2800 | 2440 | 877 |
| Platelet function test | VerifyNow P2Y12 assay HPR cutoff: ≥230 PRU | VerifyNow P2Y12 assay HPR cutoff: ≥235 PRU or ≤15% inhibition | VerifyNow P2Y12 assay HPR cutoff: ≥208 PRU, LPR cutoff: ≤85 PRU |
| Treatment type/dose in the monitoring group | Clopidogrel 600-mg loading dose, followed by 150-mg maintenance dose | Clopidogrel 600-mg loading dose plus clopidogrel 150 mg daily (−90%) or prasugrel 10 mg daily (−10%) | Prasugrel 5 mg daily (55%); prasugrel 10 mg (4%); clopidogrel 75 mg (39%) |
| Efficacy outcome (monitoring group vs conventional-treatment group) | 6-month cardiovascular death, nonfatal MI, or stent thrombosis: 2.3% vs 2.3%; HR 1.01, P = .97 | 1-year death, MI, stent thrombosis, stroke, or urgent revascularization: 34.6% vs 31.1%; HR 1.13, P = 0.10 | 1-year cardiovascular death, MI, stroke, stent thrombosis, urgent revascularization, and BARC-defined bleeding (types 2, 3, or 5): 28% vs 28%; HR 1.0, P = .98 |
| Safety outcome | Severe or moderate GUSTO bleeding: 1.4% vs 2.3%; HR = 0.59, P = .10 | Major STEEPLE bleeding:2.3% vs 3.3%; HR = 0.57, P = .12 | BARC-defined bleeding (types 2, 3, or 5): 21% vs 20%; HR 1.04, P = .77 |
| Limitations | • Low-risk patient cohort resulted in low event rate, hence underpowered to test the utility of PFM | • Low-risk patient cohort | • Uniform strategy for first 14 days: utility of PFM not tested in early period |
| • Suboptimal remedy to overcome HPR | • Suboptimal remedy to overcome H PR in majority of patients | • Therapeutic approach in the PFM group is primarily to reduce bleeding | |
| • Randomization done 12 or 24 hours after PCI, missing periprocedural events | • Underpowered for postdischarge event occurrence | • Approximately 8.7% of the PFM group did not undergo PFM on either day 14 or day 28 |
Reproduced with permission from Gurbel and Tantry.72
CAD, coronary artery disease; DES, drug-eluting stent; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; HPR, high platelet reactivity to ADP; HR, hazard ratio; LPR, low platelet reactivity to ADP; MI, myocardial infarction; NSTE-ACS, non-ST-segment elevation acute coronary artery syndrome; PFM, platelet function monitoring; PRU, P2Y12 reaction units.
Platelet function tests and bleeding
Some,58,77,78 but not other,70 studies of clopidogrel-treated patients suggest a relationship between low platelet reactivity and bleeding events. In any event, the relationship is weaker than that between high platelet reactivity and thrombotic events,78 and there is no convincing evidence that changing clopidogrel therapy based on platelet function monitoring can result in a reduction in bleeding events and some evidence that it may increase bleeding events.79
However, we perform platelet function testing to (a) confirm suspected nonadherence in patients taking clopidogrel (or other antiplatelet therapy) or to confirm suspected nonadherence in patients with ischemic events42,49 and (2) to determine the timing of cardiac surgery following withdrawal of a P2Y12 inhibitor to provide reassurance to the surgeon and allow earlier operation in patients with urgent indications but in whom the surgeon may otherwise feel obligated to wait the requisite number of days listed in the drug labeling or guidelines.80-82
CYP2C19*2 single nucleotide polymorphism
Clopidogrel is metabolized through the cytochrome P-450 family of enzymes to generate the active metabolite required for inhibition of the platelet ADP receptor P2Y12.51 The CYP2C19*2 single nucleotide polymorphism negatively affects clopidogrel pharmacokinetics and pharmacodynamics, resulting in a higher incidence of ischemic outcomes after PCI.54-56 However, in the Assessment by a Double Randomization of a Conventional Antiplatelet Strategy versus a Monitoring-Guided Strategy for Drug-Eluting Stent Implantation and, of Treatment Interruption versus Continuation One Year After Stenting-GENE study of 1394 patients scheduled for coronary stent implantation, CYP2C19*2 was unrelated to clinical outcomes.83 The CYP2C19*2 polymorphism was also unrelated to ischemic outcomes in ACS patients or stable patients.84-86 Two assays are available for the rapid identification of the CYP2C19 genotype: the Spartan RX system and the Verigene XP system. In a study of clopidogrel-treated patients after PCI, CYP2C19*2 carriers identified at the bedside were treated with prasugrel, which reduced their on-treatment platelet reactivity.87 In a small mechanistic study, among patients with stable cardiovascular disease in the Escalating Clopidogrel by Involving a Genetic Strategy-Thrombolysis In Myocardial Infarction 56 trial, tripling the maintenance dose of clopidogrel to 225 mg daily in CYP2C19*2 heterozygotes achieved levels of platelet reactivity similar to that seen with the standard 75-mg dose in noncarriers; in contrast, for CYP2C19*2 homozygotes, doses as high as 300 mg daily did not result in comparable degrees of platelet inhibition.88 However a genome-wide association study found that the CYP2C19*2 polymorphism accounted for only 12% of the variation in platelet aggregation following clopidogrel administration,55 and clopidogrel pharmacokinetics and pharmacodynamics still vary widely in patients lacking the CYP2C19*2 polymorphism.51 Therefore, attempts to use the CYP2C19*2 polymorphism as a guide to modifying clopidogrel therapy in patients would not be expected to be of clinical benefit in most clopidogrel-treated patients with high on-treatment platelet reactivity.
Summary of case 2
There is no evidence that this 55-year-old man patient, who recently had a coronary artery stent placed during a PCI, would benefit clinically by using a test to determine whether his dose of clopidogrel should be modified.
Conclusions
Available evidence does not support the use of laboratory tests to guide the dose of aspirin or clopidogrel in patients with so-called aspirin or clopidogrel resistance. However the most definitive evidence for this conclusion comes from adult patients with coronary artery disease. More evidence is needed in other disease settings in which aspirin and/or clopidogrel is used (eg, stroke, transient ischemic attacks, neurointerventions, peripheral artery disease, left ventricular assist devices). Although a number of small studies have suggested possible benefit from guided therapy in these clinical settings and in children, it is prudent to consider that highly favorable results were reported from early small randomized controlled trials73 in patients with coronary artery disease before much larger, more definitive randomized controlled trials failed to confirm any benefit from this approach.74-76 There is a possible role for platelet function testing (1) to document patient adherence to antiplatelet therapy or to confirm suspected nonadherence in patients with ischemic events42,49 and (2) to determine the timing of cardiac surgery following withdrawal of a P2Y12 inhibitor to provide reassurance to the surgeon and allow earlier operation in patients with urgent indications but in whom the surgeon may otherwise feel obligated to wait the requisite number of days listed in the drug labeling or guidelines.80-82 Although research studies are still ongoing, there is no current clinical role for routine platelet function or genetic testing for antiplatelet resistance.
Authorship
Contribution: A.D.M. and D.L.B. wrote the paper.
Conflict-of-interest disclosure: A.D.M.: scientific advisory committees for AstraZeneca, Instrumentation Laboratory, Janssen, Lilly, and Momenta; research funding from Anelixis, Baxalta, Eisai, GE Global Research, HeartWare/Medtronic, Megakaryon, Pfizer, and Sysmex; and international patent WO 2016/191332 A2. D.L.B.: advisory board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; board of directors: Boston Veterans Administration (VA) Research Institute and Society of Cardiovascular Patient Care; chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, and Population Health Research Institute; honoraria: American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org), Belvoir Publications (editor in chief, Harvard Heart Letter), Duke Clinical Research Institute and Harvard Clinical Research Institute (clinical trial steering committees), HMP Communications (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor; associate editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (chief medical editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (continuing medical education steering committees); other: Clinical Cardiology (deputy editor), National Cardiovascular Data Registry-ACTION Registry Steering Committee (chair), VA Cardiovascular Assessment, Reporting, and Tracking Research and Publications Committee (chair); research funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, and The Medicines Company; royalties: Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site coinvestigator: Biotronik, Boston Scientific, and St. Jude Medical; trustee: American College of Cardiology; and unfunded research: FlowCo, Merck, PLx Pharma, and Takeda.
Correspondence: Alan D. Michelson, Boston Children’s Hospital, Karp 08213, 300 Longwood Ave, Boston, MA 02115; e-mail: alan.michelson@childrens.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal