Abstract
Central nervous system (CNS) relapses are an uncommon yet devastating complication of non-Hodgkin lymphomas. The identification of patients at high risk of secondary CNS relapse is therefore paramount. Retrospective data indicate prophylactic CNS-directed therapies may reduce the risk of CNS involvement; however, no consensus exists about dose, timing, or route of therapy. In addition, prophylaxis is not without risk of treatment-related complications and morbidity. Here, we present a series of case vignettes highlighting our approach to common dilemmas encountered in routine clinical practice. We review the method of assessing CNS relapse risk, factors that increase the likelihood of relapse including histologic subtype, MYC rearrangement, protein expression, and extranodal involvement, and review our clinical practice based on available evidence in administering CNS-directed prophylaxis.
Introduction
Non-Hodgkin lymphomas are biologically and clinically diverse hematological malignancies. Treatment is influenced by patient fitness, disease biology, and tumor burden. Identifying patients at high risk of central nervous system (CNS) relapse is important as the outcome of secondary CNS lymphoma is poor.1-4 Risk of CNS progression is influenced by histologic subtype and subtype-specific clinicopathologic features (eg, site of involvement, protein expression, or gene rearrangements). Patients with highly aggressive lymphomas (eg, lymphoblastic, Burkitt lymphoma) are at high risk and frontline protocols include CNS-directed prophylaxis. In contrast, indolent lymphomas rarely involve the CNS and CNS prophylaxis is not required. Between these extremes fall diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma (HGBL) with MYC and BCL2 or BCL6 rearrangements, so-called “double-hit” lymphomas (HGBL-DH), and (nodal) peripheral T-cell lymphomas (PTCLs). The addition of rituximab has slightly reduced CNS relapse in DLBCL, probably through superior systemic control as there is negligible CNS penetration of the drug across the intact blood-brain barrier.1,5 However, ∼4% of unselected patients with DLBCL treated with prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (R-CHOP) experience CNS relapse,6,7 and considerable efforts have been made to identify those at greatest risk. In this review, we will summarize our approach to this problem with case vignettes drawn from our practice.
How I identify patients at increased risk for CNS relapse
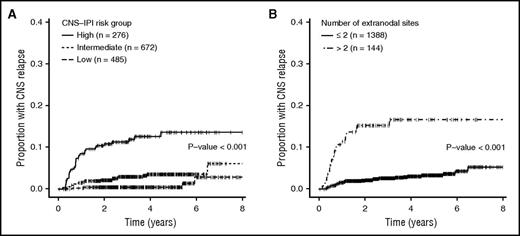
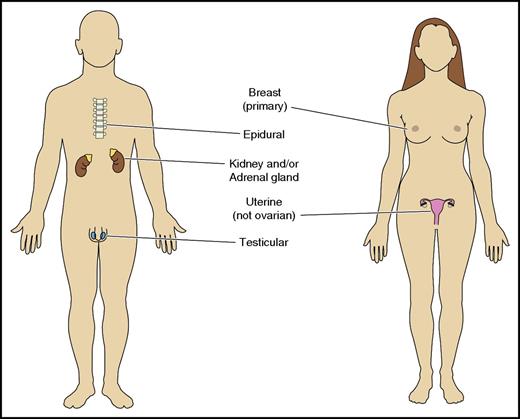
Many investigators have examined risk factors for CNS relapse, but studies have yielded inconsistent results, due to heterogeneity in patient populations, treatment, and/or limited sample size.8-16 To address some of these limitations, Schmitz et al developed a 6-point score from 2164 patients treated on prospective German studies and validated in 1597 patients with DLBCL treated with R-CHOP in the British Columbia Cancer Agency (BCCA) Lymphoid Malignancies Database.6 The components of the score were the International Prognostic Index (IPI) factors: age > 60 years, elevated serum lactate dehydrogenase (LDH), Eastern Cooperative Oncology Group (ECOG) status > 1, stage III/IV, >1 extranodal site, with the addition of kidney/adrenal involvement. The resulting “CNS-IPI” separates patients into low- (0-1 factor), intermediate- (2-3), and high- (4+) risk groups with 2-year CNS relapse rates of 0.6%, 3.4%, and 10.2%, respectively. This provides a robust and readily calculable risk estimate in patients with DLBCL that has been externally validated in another independent cohort of 1532 patients with similar results (Figure 1A).7 Despite its strengths, it remains imperfect with low positive predictive value (10%-12%). Thus, if used to select patients for prophylaxis, most patients unnecessarily receive CNS prophylaxis. Other factors including the involvement of other specific anatomic sites (Figure 2) and biologic factors have been identified in separate studies as predictive of CNS relapse and warrant specific consideration. A summary of the factors we consider when assessing CNS risk is presented in Table 1.6,16-29
Cumulative incidence of CNS relapse among 1532 patients with DLBCL. (A) Patients were treated with R-CHOP–like regimens according to CNS-IPI and (B) number of extranodal sites was determined by PET-CT. Reprinted from El-Galaly et al7 with permission.
Cumulative incidence of CNS relapse among 1532 patients with DLBCL. (A) Patients were treated with R-CHOP–like regimens according to CNS-IPI and (B) number of extranodal sites was determined by PET-CT. Reprinted from El-Galaly et al7 with permission.
Specific extranodal sites associated with increased risk of CNS relapse.
Risk stratification for secondary CNS involvement in non-Hodgkin lymphoma integrating histologic, clinical, and molecular factors
| Histologic subtype . | Histologic subtype-specific risk factors . | Approximate CNS relapse risk . |
|---|---|---|
| DLBCL | CNS-IPI ≥ 46 | 10% at 2 y |
| or involvement of breast,17 testis,18 uterus,19 epidural,20,21 kidney/adrenals6,16 | Varies by site | |
| MYC/BCL2 DE DLBCL, particularly if ABC subtype22 | 10% at 2 y (15% if ABC COO) | |
| CD5+ DLBCL23 | 12.7% at 2 y | |
| Intravascular large B-cell lymphoma24 | 25% at 3 y | |
| IgM-secreting DLBCL25 | 41% cumulative incidence (7 of 17) | |
| HGBL with MYC and BCL2 and/or BCL6 rearrangements26 | 13% at 3 y | |
| MCL | Blastoid histology or Ki-67 ≥ 30%27 | 25.4% at 2 y |
| PTCL (PTCL-NOS, AITL, ALCL) | >1 extranodal site, skin or gastrointestinal involvement28 | ∼10% at 2 y |
| ALK+ ALCL | >1 extranodal site29 | 1-y 15% |
| Histologic subtype . | Histologic subtype-specific risk factors . | Approximate CNS relapse risk . |
|---|---|---|
| DLBCL | CNS-IPI ≥ 46 | 10% at 2 y |
| or involvement of breast,17 testis,18 uterus,19 epidural,20,21 kidney/adrenals6,16 | Varies by site | |
| MYC/BCL2 DE DLBCL, particularly if ABC subtype22 | 10% at 2 y (15% if ABC COO) | |
| CD5+ DLBCL23 | 12.7% at 2 y | |
| Intravascular large B-cell lymphoma24 | 25% at 3 y | |
| IgM-secreting DLBCL25 | 41% cumulative incidence (7 of 17) | |
| HGBL with MYC and BCL2 and/or BCL6 rearrangements26 | 13% at 3 y | |
| MCL | Blastoid histology or Ki-67 ≥ 30%27 | 25.4% at 2 y |
| PTCL (PTCL-NOS, AITL, ALCL) | >1 extranodal site, skin or gastrointestinal involvement28 | ∼10% at 2 y |
| ALK+ ALCL | >1 extranodal site29 | 1-y 15% |
ABC, activated B cell; AITL, angiommunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; HGBL, high-grade B-cell lymphoma; IgM, immunoglobulin M; MCL, mantle cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified.
Case 1
Presentation.
A 78-year-old retired nurse presented with hematemesis. At endoscopy, a gastric tumor was biopsied and showed non–germinal center B-cell (GCB) DLBCL (by Hans algorithm).30 A staging positron emission tomography with computed tomography (PET-CT) scan identified widespread lymphadenopathy and involvement of the adrenal glands, kidneys, and bone. She did not have signs or symptoms suggestive of CNS involvement. Her ECOG was 2 and LDH elevated (IPI = 5, CNS-IPI = 6). Examination of the blood film and peripheral blood lymphocyte immunophenotyping excluded circulating lymphoma cells. Cerebrospinal fluid (CSF) cytology (CC) and flow cytometry (FCM) were negative for lymphoma.
Discussion.
The decision to offer CNS prophylaxis to this patient was straightforward. Her predicted 2-year CNS risk of 10.2% using the CNS-IPI model was likely an underestimate. In fact, for the 0.6% of patients with the maximum CNS-IPI score of 6, the 2-year risk of CNS relapse was 32.5%.6 We always send CSF for CC and FCM as the use of FCM increases the sensitivity of detecting lymphoma in this compartment.31 Occult leptomeningeal disease (CC−/FCM+) is associated with markedly increased risk of frank CNS progression and warrants aggressive CNS-directed therapy.32,33
Outcome.
We commenced prephase prednisone followed by R-CHOP given every 21 days (R-CHOP21) for 6 cycles with 1 dose of intrathecal methotrexate (IT MTX) per cycle. In addition, 2 cycles of systemic MTX were administered (with rituximab, total of 8 doses) at a reduced dose of 1 g/m2 due to her age. She achieved complete metabolic response (CMR) at the end of treatment, but unfortunately experienced nodal disease relapse with B symptoms and retroperitoneal and mediastinal lymphadenopathy 9 months later. Her disease was refractory to second-line chemotherapy. She was offered participation in a clinical trial and remains on study with stable disease after 4 months on the investigational agent.
Case 2
Presentation.
A 45-year-old female lawyer presented with a painless right breast lump and subsequently underwent a core biopsy. Histopathology confirmed DLBCL with non-GCB phenotype (by Hans algorithm). The contralateral breast was normal by clinical examination and ultrasound; PET-CT confirmed ipsilateral breast involvement only (stage IAE or primary breast lymphoma). Her ECOG status was 0, LDH normal (IPI = 0 and CNS-IPI = 0).
Discussion.
Certain anatomic sites of extranodal involvement of DLBCL are strongly associated with CNS relapse, even when the CNS-IPI is low.34 Although the kidney/adrenals were independent predictors in the CNS-IPI, extranodal sites identified in other datasets (epidural, breast, uterus, and testes) were not. This is probably explained by underrepresentation of such patients from prospective studies. For instance, many patients with epidural involvement require emergent radiotherapy to treat impending spinal cord compression precluding clinical trial participation, and patients with stage IE lymphomas are excluded from many protocols. The propensity for primary testicular lymphoma to disseminate to the CNS is well described18,35,36 ; a specific treatment protocol was shown in a prospective phase 2 study to result in an apparent reduction in CNS risk relative to historic controls.37 In contrast, the association between primary breast lymphoma and CNS relapse is underappreciated, despite data suggesting crude incidence of 12% to 16%.17,38,39 Stage IIE disease,17 stage-modified IPI ≥ 2,17 bilateral breast involvement,40 and tumor > 5 cm41 have all been observed in individual studies to be potential risk factors for CNS involvement, but the findings have not been consistently replicated. Epidural involvement was associated with increased CNS risk in prerituximab case series,42-45 however, contemporary data are lacking. Sinus involvement was associated with marginally increased CNS risk (6% in the prerituximab era) which was reduced to 1.6% when rituximab was incorporated into primary therapy.46,47 El-Galaly et al identified a strong association between uterine (but not ovarian) involvement with DLBCL and CNS risk (hazard ratio, 14.1) by multivariate analysis, independent of the CNS-IPI.19 We offer patients with breast, uterine, testicular, and epidural involvement CNS prophylaxis, irrespective of their CNS-IPI.
Outcome.
Despite the CNS-IPI of 0, we treated this patient with 6 cycles of R-CHOP21 with IT MTX and 2 cycles of high-dose MTX (HD-MTX; 3 g/m2). Consolidative radiotherapy was delivered to the ipsilateral breast and she remains in ongoing remission 4 years from initial presentation.
Case 3
Presentation.
A 68-year-old retired truck driver with comorbidities including chronic obstructive pulmonary disease and ischemic cardiomyopathy (ejection fraction, 35%) presented with asymptomatic lymphadenopathy. Biopsy showed both grade 3B follicular and DLBCL in the same specimen. PET-CT identified widespread lymphadenopathy with bone marrow the only apparent site of extranodal involvement. He had no antecedent history of indolent lymphoma. The ECOG was 0 and LDH normal. He therefore had stage IVA composite lymphoma (IPI = 2, CNS-IPI = 2). However, fluorescence in situ hybridization confirmed rearrangements in MYC, BCL2, and BCL6, that is, “triple-hit” lymphoma. CSF was negative for lymphoma (CC−/FCM−).
Discussion.
MYC-rearranged non-Burkitt lymphomas have aggressive behavior and poor outcomes with R-CHOP.48 Aggressive lymphomas bearing rearrangements in MYC and BCL2 and/or BCL6 were previously termed “double-hit” lymphomas (DHLs) or “triple-hit” lymphomas with a spectrum in morphology from DLBCL to Burkitt lymphoma. In the World Health Organization (WHO) 2008 classification, many were captured under the provisional entity of “B-cell lymphoma, unclassifiable (BCL-U), with features intermediate between DLCBL and Burkitt lymphoma.”49 In the 2017 update, all aggressive lymphomas (except those with lymphoblastic or follicular lymphoma morphology) bearing rearrangements in MYC and BCL2 and/or BCL6 were reclassified as “high-grade B-cell lymphoma, with MYC and/or BCL2 or BCL6 rearrangements” (HGBL-DH). Although early studies of patients with DLBCL bearing MYC and/or BCL2 rearrangements indicated markedly increased risk of CNS involvement at diagnosis of up to 44%,50,51 2 larger retrospective studies indicated that 4% to 7% of patients had CNS involvement at diagnosis with a 3-year cumulative CNS risk of 13%.26,52 Both studies also suggested use of CNS prophylaxis may improve outcomes: in the first, IT MTX prophylaxis was associated with a reduction in CNS progression (3-year incidence, 5% v 15%; P = .017)26 ; in the second, use of CNS prophylaxis was associated with improvement in overall survival.52 We therefore consider HGBL-DH at high risk of CNS involvement and consider these patients for CNS-directed prophylaxis. The 2017 WHO categories of HGBL-DH and HGBL not otherwise specified (HGBL-NOS) have created difficulty in applying evidence from older datasets (based on the superseded BCL-U). We are unaware of specific data examining the risk of CNS progression in HGBL-NOS (ie, without MYC translocations). However, we continue to recommend CNS prophylaxis for patients with HGBL-NOS based on the increased risk observed in BCL-U and Burkitt lymphoma; in retrospective series, many patients with BCL-U morphology presented with IPI 3 to 5 even in the absence of MYC translocations.53,54 However, this issue clearly warrants further study in larger datasets with central pathology review.
Outcome.
We favor dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) for fit patients with HGBL-DH based on retrospective26,52,55 and prospective data.56 However, given substantial comorbidities including cardiac dysfunction, we used 6 cycles of cyclophosphamide, etoposide, prednisone, vincristine, and rituximab (R-CEOP; anthracycline substituted for etoposide). We administered IT MTX 12 mg with each cycle of the first 4 cycles of chemoimmunotherapy. After the fourth cycle, he developed severe post–lumbar puncture headache unresponsive to simple analgesia and caffeine. As all CSF assessments had been negative, further IT prophylaxis was abandoned. On completion of chemoimmunotherapy, after 2 cycles of HD-MTX (3 g/m2) in addition with rituximab (to complete 8 doses), the end of treatment PET-CT demonstrated a “near” CMR (Deauville 3, with residual low-grade uptake at a periportal node unsafe to rebiopsy). The node remains unchanged in size and avidity 3 months later and he remains in clinical remission at 8 months follow-up.
Case 4
Presentation.
A 67-year-old male office worker presented with abdominal discomfort, night sweats, and hypercalcemia. CT-guided core biopsy of retroperitoneal lymphadenopathy showed DLBCL with non-GCB phenotype (by Hans algorithm); cells expressed both MYC (70%) and BCL2 (60%) by immunohistochemistry. MYC–fluorescence in situ hybridization was negative. PET-CT revealed extensive nodal disease and involvement of the liver, multiple bone lesions, and muscle. CSF analysis was negative. His stage was IVB, ECOG was 0, and serum LDH (surprisingly) was not elevated (IPI = 3, CNS-IPI = 3).
Discussion.
Although not fulfilling CNS-IPI criteria for high risk, we assessed the patient to be at high risk of CNS relapse for 2 reasons. First, data from Vancouver suggest MYC and BCL2 protein dual expressers (DEs) are at increased risk of CNS relapse. Savage et al examined the correlation between DE status, cell of origin (COO; Lymph2Cx nanostring57 and Hans algorithm), and CNS relapse in 428 patients with de novo DLBCL treated with R-CHOP (largely without CNS prophylaxis).22 DE patients were more likely to experience CNS relapse than non-DE (2-year CNS relapse risk, 9.7% v 2.2%; P = .001). Activated B-cell (ABC) COO was also associated with increased CNS relapse risk (9.4% v 2.2%; P = .02). However, by multivariate analysis, only DE status (hazard ratio, 3.68) and CNS-IPI (hazard ratio, 5.21) remained significant predictors of CNS relapse.22 Patients whose tumors were both DE and ABC COO had a 2-year CNS relapse risk of 15.3%. The study was important as DEs account for around 30% of DLBCL (around 6 times more frequent than MYC/BCL2-rearranged lymphomas)48,58,59 and around two-thirds of DE are ABC COO. In contrast, HGBL-DH typically arise in tumors with GCB phenotype, acknowledging that COO nomenclature is limited to pure cases of DLBCL in the revised 2017 WHO category.60-62
The second reason we would have administered CNS prophylaxis independent of DE status is the involvement of 3 extranodal sites on PET-CT. El-Galaly et al studied an independent cohort of 1532 DLBCL patients staged with PET-CT and treated with R-CHOP21 (or similar) and observed a striking correlation between the absolute number of extranodal sites and risk of CNS progression.7 Multiple areas of involvement within 1 organ or tissue (eg, multifocal bone involvement) only counted for 1 site. Patients with ≥3 extranodal sites comprised 9.5% of the cohort and had a 2-year CNS relapse risk of 15.2% (Figure 1B). The authors explored the “≥3 extranodal sites” model further, and compared with the CNS-IPI, it identified fewer patients as high risk (9.5% vs 19.2%; P = .005) and as a result was less sensitive (35.5% vs 55.7%; P < .001), more specific (91.7% vs 82.3%; P < .001), and more accurate (89.4% vs 81.2%; P = .001), but had similar positive predictive value (15.3% vs 11.2%; P = .1). Although both models offer an excellent negative predictive value (∼97%), the ≥3 extranodal sites model has the potential advantages of being easier to remember and resulting in fewer patients being exposed to CNS prophylaxis (at the cost of decreased sensitivity). The model should be validated in an external cohort; however, at present, we consider patients with ≥3 extranodal sites for CNS prophylaxis even if they are not “high risk” by CNS-IPI.
Outcome.
Based on DE status and involvement of ≥3 extranodal sites, we estimated the patient’s 2-year risk of CNS relapse to be ∼15% despite the CNS-IPI score of 3 (intermediate). We commenced treatment with DA-EPOCH-R with 1 dose of IT MTX per cycle, followed by 2 cycles of systemic MTX following the completion of chemoimmunotherapy. End of treatment PET-CT demonstrated CMR and remains in remission 16 months after completion of therapy. We should highlight that in contrast to DHL, there are fewer data supporting DA-EPOCH-R in DE lymphoma. A small retrospective series from MD Anderson Cancer Center (MDACC) suggested a potential benefit,63 however, this remains to be confirmed in larger, prospective series. Furthermore, overall results from the CALGB 50303 study (the only phase 3 randomized comparison of R-CHOP vs DA-EPOCH-R) was negative for cohort overall, although results from biologic subgroups including COO, DEs, and DHLs are awaited.64
Case 5
Presentation.
A 32-year-old engineer presented with widespread lymphadenopathy and fevers. Biopsy showed anaplastic lymphoma kinase–positive (ALK+) anaplastic large cell lymphoma (ALCL). PET-CT identified involvement of the liver, spleen, and multifocal bone lesions. This patient had stage IVB disease, ECOG of 1, and elevated LDH (IPI = 3, CNS-IPI = 3). Treatment with cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone (CHOEP) was commenced at another institution. He attended our institution for a second opinion after completing the first cycle of therapy.
Discussion.
Applying the CNS-IPI (derived largely from patients with DLBCL), the risk would be intermediate (∼3%), not warranting CNS-directed prophylaxis. However, investigators have studied PTCL separate from DLBCL to better refine risk factors for CNS involvement. Ellin et al reported that 28 of 625 patients with PTCL (4.5%) in a Swedish population-based study developed CNS relapse with >1 extranodal site, skin, and gastrointestinal involvement associated with CNS risk by multivariate analysis.28 In a Korean study of 228 patients, Yi et al identified elevated LDH and paranasal sinus involvement as associated with CNS relapse.65 In both studies, all PTCL subtypes were considered together to derive risk factors. Of specific relevance to this patient, Chihara et al analyzed CNS risk according to PTCL subtype in 616 patients treated at MDACC: among 76 patients with ALK+ ALCL, the 5-year risk of CNS relapse was 5.3%; however, among ALK+ ALCL and >1 extranodal site the 1-year CNS relapse was 15%.29 Due to the rarity and heterogeneity of PTCL, interpretation of these studies presents a challenge, but the involvement of multiple extranodal sites appears a recurrent risk factor. Therefore, for this patient with ALK+ ALCL and 2 extranodal sites (liver and bone; we do not consider spleen to be extranodal for the purposes of determining CNS risk), we estimated the 1-year CNS risk for this patient to be 15%.
Mantle cell lymphoma (MCL) is another aggressive histologic subtype where the role of CNS prophylaxis remains controversial. Unselected patients with MCL have an estimated CNS relapse rate of 5.4%, with high-risk features including Ki-67 >30% associated with increased risk (hazard ratio, 6.03; P = .003).27 There are limited data supporting a role for CNS prophylaxis. Outside of clinical trials, in transplant-eligible patients we use high-dose cytarabine-based induction followed by autologous stem cell transplant; in elderly patients, we use bendamustine-rituximab, but we do not specifically add CNS-directed prophylaxis in either setting. However, most patients with MCL at our institution are offered participation in investigational protocols incorporating Bruton tyrosine kinase inhibitors; there are data suggesting the first-in-class agent, ibrutinib, penetrates the CNS66 and may be effective in MCL with CNS involvement.67 We have not observed any CNS relapses in our MCL patients treated with Bruton tyrosine kinase inhibitors (C.Y.C., pooled analysis of patients with MCL treated on phase 1-3 studies with BTK inhibitors as single agents or in combination with chemotherapy, unpublished data). However, larger datasets are required to confirm this observation.
Outcome.
Although there are scarce data among patients with PTCL, by extrapolation from DLBCL we added IT MTX with each cycle of chemotherapy followed by 2 cycles of systemic MTX at the completion of CHOEP. This patient achieved a CMR at the completion of therapy and remains in remission at 12 months follow-up.
How I deliver CNS prophylaxis
The optimal method for administration of CNS-directed prophylaxis is unknown. Even among high-risk patients, only a minority develop CNS recurrence, making adequately powered prospective randomized studies to address this question challenging. The regimen we have outlined is adapted from an approach developed at the Peter MacCallum Cancer Centre, Melbourne, Australia.68 IT MTX is administered once per chemoimmunotherapy cycle (total of 6). Three to 4 weeks after the completion of chemoimmunotherapy, 2 cycles of systemic MTX are administered, 2 to 3 weeks apart. We discontinue medications that may interfere with MTX clearance (eg, cotrimoxazole, proton pump inhibitors) at least 3 days prior to admission for IV MTX. We admit patients the afternoon before scheduled MTX and alkalinize the urine with IV fluids containing sodium bicarbonate, with careful attention to clinical assessment of fluid status (weighing patients twice daily) and using diuretics as needed to prevent fluid overload. We administer MTX at a target dose of 3 g/m2 over 4 hours with leucovorin rescue commencing 24 hours later. In patients with mild to moderate renal impairment, or those aged >70 years, we reduce the dose of IV MTX to 1 g/m2 to 1.5 g/m2.
IT MTX as CNS prophylaxis in aggressive lymphoma was extrapolated from acute lymphoblastic leukemia, where CNS recurrence is usually leptomeningeal. In contrast, CNS relapse in DLBCL usually has a parenchymal component4 and the limited ability of cytotoxic drugs administered by IT injection to penetrate into deep brain tissue is problematic, with data suggesting minimal impact on CNS progression.1,13,69,70 Systemic therapy with CNS penetration is therefore paramount and an essential component for all prophylactic regimens. Systemic HD-MTX achieves tumoricidal levels in brain parenchyma at doses ≥1 g/m2 and leptomeningeal penetration at doses ≥3 g/m2.71-74 A French randomized study (prerituximab) compared doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone with a consolidation phase containing etoposide, ifosfamide, cytarabine, 4 doses of IT MTX, and 2 cycles of systemic MTX 3 g/m2 (ACVBP) with CHOP (which contained no CNS prophylaxis).75 The CNS relapse rate was lower in the arm treated with IT and IV MTX (2.7% vs 8%; P = .02), acknowledging that other CNS-penetrating agents were used in the MTX arm. Several subsequent retrospective68,76,77 and 2 prospective studies also support a potential benefit for high-dose systemic MTX in this setting.78,79 However, these studies are limited by small numbers and nonrandomized and retrospective study designs, with the prospective studies using other CNS-penetrating agents in combination with MTX. There remains lack of consensus regarding a systemic MTX-dosing regimen, number of cycles, and whether IT prophylaxis has any role or not. No randomized study exists to show that IT prophylaxis is effective, with only small retrospective, single-arm, or nonrandomized studies in combination with systemic therapy providing limited evidence for a reduction in CNS risk.10,37,80,81 Given that 20% of CNS progression occurs during primary therapy,3 delaying all CNS-directed measures until completion of chemoimmunotherapy is too late. Accordingly, some groups are using systemic MTX either before chemoimmunotherapy or intercalated between cycles. Although this is entirely rational with regard to prevention of early CNS progression, high-dose systemic MTX can be associated with toxicity, most notably renal impairment, which occurs in up to 9% of cycles.82 This could potentially interrupt the primary (curative intent) chemoimmunotherapy.83 We recognize that the quality of data supporting these approaches are suboptimal and prospective studies should guide the ideal strategy.
It is noteworthy that several groups have explored dose-intensified regimens for younger patients with DLBCL and poor prognostic features in nonrandomized studies. Dunleavy et al used DA-EPOCH-R with IT MTX as the sole form of CNS prophylaxis in 52 patients with aggressive MYC-rearranged B-cell lymphoma, 65% with IPI ≥ 3.56 To date, no CNS progressions have occurred, though final results are awaited (K. Dunleavy, George Washington University, e-mail, March 2016). The numbers of patients treated remain relatively small, though the CNS progression rate in the phase 2 study using the same regimen in low-risk Burkitt lymphoma appeared to prevent CNS relapse.84 In contrast, investigators from Chicago retrospectively examined 117 patients with DLBCL treated with DA-EPOCH-R, 62 of whom received IT MTX and 55 of whom did not.85 The crude incidence of CNS relapse was 7 of 117 (6%) and IT MTX did not appear to be associated with reduction in risk. Limitations of retrospective design notwithstanding, these data highlight that CNS progression in patients receiving DA-EPOCH-R and IT MTX can occur. When we use DA-EPOCH-R, if CNS prophylaxis is indicated, we add 2 cycles of high-dose IV MTX after the last cycle of chemoimmunotherapy, as in case 4. This shares some similarities with the Nordic approach. Holte et al used dose-dense rituximab, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone delivered in 14-day cycles, followed by intensification with high-dose cytarabine and HD-MTX in 156 patients with DLBCL aged 18 to 65 years with age-adjusted IPI 2-3.79 Apart from 1 dose of IT MTX on baseline lumbar puncture, IT chemotherapy prophylaxis was not given. CNS relapses occurred in 7 patients (4.4%), all within 6 months of study entry, again highlighting the need to provide some form of prophylaxis early during treatment. Finally, Phillips et al reported CNS outcomes from the UK National Cancer Research Institute phase 2 study of rituximab, cyclophosphamide, doxorubicin, vincristine, high-dose methotrexate, followed by ifosfamide, etoposide, high-dose cytarabine, rituximab with intrathecal methotrexate and cytarabine in intermediate-high risk DLBCL.78 Among the 55 patients with high-risk CNS-IPI, the 2-year CNS relapse rate was lower than expected at 6.2%. This observation suggests a potential benefit from the early inclusion of HD-MTX used in this regimen, however, confirmation of this finding in larger, randomized studies is needed.
Conclusion and future directions
Although the case vignettes detailing our approach to CNS prophylaxis have been successful, it is important to acknowledge that failures may still occur. This highlights the need for further studies to better identify high-risk patients; better prophylactic strategies are needed as well. Novel agents such as ibrutinib and lenalidomide cross the blood-brain barrier67,86 and are active in both systemic ABC DLBCL87,88 and CNS lymphomas.89-91 In a recent pooled analysis of 2 prospective studies using R-CHOP plus lenalidomide in 136 patients (18% CNS-IP I ≥ 4) with a median follow-up of 48 months, only 1 patient (0.7%) developed isolated CNS relapse despite minimal use of CNS prophylaxis with IT (15%) or IV (0%) MTX.92 Randomized phase 3 studies comparing R-CHOP ± ibrutinib (NCT01855750) and R-CHOP ± lenalidomide (NCT01856192; NCT02285062) in DLBCL will hopefully answer the question of whether these agents can replace existing CNS prophylactic strategies.
Until then, we suggest that careful assessment for CNS recurrence be integrated into routine therapeutic decision-making for patients with aggressive lymphomas.
Acknowledgment
C.Y.C. acknowledges the mentorship of John Seymour over many years, particularly in relation to the subject of this review.
Authorship
Contribution: C.K.C. performed the literature review and wrote the first draft of the manuscript, and C.Y.C. designed the paper and reviewed and cowrote the manuscript.
Conflict-of-interest disclosure: C.Y.C. received research funding from Celgene and Roche; was on the speakers bureau for Roche, Janssen-Cilag, and Takeda; was an advisory board member for Janssen-Cilag and Bristol-Myers Squibb; and received travel expenses from Bristol-Myers Squibb. C.K.C. declares no competing financial interests.
Correspondence: Chan Yoon Cheah, Department of Haematology, Sir Charles Gairdner Hospital, Ground Floor, B Block, Hospital Ave, Nedlands, WA 6009, Australia; e-mail: chan.cheah@health.wa.gov.au.