Key Points
Inherited ciHHV-6 was detected in 1.4% of HCT recipients and 0.9% of their donors.
Acute GVHD grades 2-4 and cytomegalovirus viremia were more frequent when recipients or donors had inherited ciHHV-6.
Abstract
Human herpesvirus 6 (HHV-6) species have a unique ability to integrate into chromosomal telomeres. Mendelian inheritance via gametocyte integration results in HHV-6 in every nucleated cell. The epidemiology and clinical effect of inherited chromosomally integrated HHV-6 (iciHHV-6) in hematopoietic cell transplant (HCT) recipients is unclear. We identified 4319 HCT donor–recipient pairs (8638 subjects) who received an allogeneic HCT and had archived pre-HCT peripheral blood mononuclear cell samples. We screened these samples for iciHHV-6 and compared characteristics of HCT recipients and donors with iciHHV-6 with those of recipients and donors without iciHHV-6, respectively. We calculated Kaplan-Meier probability estimates and Cox proportional hazards models for post-HCT outcomes based on recipient and donor iciHHV-6 status. We identified 60 HCT recipients (1.4%) and 40 donors (0.9%) with iciHHV-6; both recipient and donor harbored iciHHV-6 in 13 HCTs. Thus, there were 87 HCTs (2%) in which the recipient, donor, or both harbored iciHHV-6. Acute graft-versus-host disease (GVHD) grades 2-4 was more frequent when recipients or donors had iciHHV-6 (adjusted hazard ratios, 1.7-1.9; P = .004-.001). Cytomegalovirus viremia (any and high-level) was more frequent among recipients with iciHHV-6 (adjusted HRs, 1.7-3.1; P = .001-.040). Inherited ciHHV-6 status did not significantly affect risk for chronic GVHD, hematopoietic cell engraftment, overall mortality, or nonrelapse mortality. Screening for iciHHV-6 could guide donor selection and post-HCT risk stratification and treatment. Further study is needed to replicate these findings and identify potential mechanisms.
Introduction
Human herpesvirus 6A (HHV-6A) and HHV-6B are distinct species of Roseolovirus in the β herpesvirus family. HHV-6B infects most children within the first few years of life and is the cause of roseola.1,2 HHV-6B reactivation based on DNA detection in serum or plasma occurs in 30% to 50% of allogeneic hematopoietic cell transplant (HCT) recipients, is the most frequent cause of infectious encephalitis, and is associated with acute graft-versus-host disease (GVHD), cytopenias, cytomegalovirus (CMV) reactivation, and increased overall mortality.3 The epidemiology and clinical consequences of HHV-6A are less well understood.
After primary infection, HHV-6A and HHV-6B establish latent infection in various cell types including lymphoid and myeloid cell lines.4 Whereas other human herpesviruses maintain their genome as a circular episome in latently infected cells, HHV-6A and HHV-6B have a unique ability to integrate into human chromosomes in the subtelomeric region.5 If viral integration occurs in a germline cell, vertical transmission of chromosomally integrated HHV-6 (ciHHV-6) results in offspring with a copy of the HHV-6 genome in all nucleated cells, and these individuals will pass the condition to half of their offspring through Mendelian inheritance (inherited ciHHV-6 [iciHHV-6]). Population-based studies estimate that ∼1% of the worldwide population (∼70 million people) has iciHHV-6.6
Importantly, chromosomal integration is not a virologic dead end. In vitro and in vivo studies have demonstrated lytic viral reactivation5,7 and gene transcription5,8,9 from the iciHHV-6 reservoir. The effect of iciHHV-6 on human disease is largely unknown, given the challenge of studying relatively rare conditions. One large registry study of 19 597 adults found that angina pectoris occurred 3 times more frequently among individuals with iciHHV-6,10 perhaps through telomeric disruption or viral gene expression stimulating chronic inflammation.
HCT is a unique setting in which immunosuppression allows for frequent reactivation of latent viruses.11 Inherited ciHHV-6 in the HCT recipient or donor provide challenges in identifying viral reactivation,12,13 and small studies in this population have not demonstrated any clear complication associated with iciHHV-6.14,15 We developed an efficient screening strategy16 and used a unique biorepository to perform a large-scale study of the epidemiology and clinical implications of iciHHV-6 among HCT recipients and their donors.
Methods
The protocol was approved by the Fred Hutchinson Cancer Research Center (Fred Hutch) Institutional Review Board.
Subjects and specimens
We identified a cohort of HCT recipients who underwent first allogeneic HCT at Fred Hutch from 1992 to 2013 and had leftover cellular samples available for testing. Only complete donor–recipient pairs were used for this study. Specimens were obtained from the Fred Hutch Research Cell Bank, which prospectively collects and cryopreserves peripheral blood mononuclear cells (PBMCs) from donors and recipients. Most samples used for this study were generated by infection of PBMCs with Epstein-Barr virus in vitro to create immortalized β lymphoblastoid cell lines. Samples were stored at −80°C until used. All samples were collected before HCT, when HHV-6 DNA detection resulting from viral reactivation is rare,17 and most likely a result of iciHHV-6.
Laboratory assays
HHV-6 DNA targets in genomic DNA extracted from β lymphoblastoid cell lines were amplified and detected using a real-time fluorescent probe quantitative polymerase chain reaction (qPCR) assay, as previously described.18 A conserved region of the U67/68 gene was amplified to distinguish between species HHV-6A and HHV-6B.13 We used droplet digital (dd)PCR to determine the ratio of HHV-6 DNA to human cells (using human ribonuclease P DNA [RPP30], a reference gene for cell count).13,16,19 A ratio of 1 ± 0.07 was considered positive for iciHHV-6 and distinguished between iciHHV-6 and naturally acquired infection. This assay was validated against a fluorescence in situ hybridization-confirmed iciHHV-6 cell line.
Screening algorithm for iciHHV-6
We designed a group testing approach using specimen pooling coupled with qPCR and ddPCR to screen extracted DNA for iciHHV-6, as previously described and validated.16 This approach leveraged the relatively low population prevalence of iciHHV-6 and high individual viral burden among affected individuals to efficiently identify iciHHV-6. Briefly, approximately 5 µg DNA per sample was aliquoted in 5 µL with the addition of 20 µL buffer AE (Qiagen, CA) (0.2 µg/µL). We pooled 2 µL from each of 12 samples in PCR plate wells and tested 10 µL per pool for HHV-6 DNA with qPCR (0.2 µg/µL × 10 = 2 µg for PCR). Thus, each patient sample contributed 0.17 µg DNA (2 µg/12 samples = 0.17 µg per sample) and approximately 2.5 × 104 cells per PCR reaction, given that 1 µg cellular DNA represents approximately 1.5 × 105 cells. Individual samples from high-positive pools (>103 HHV-6 DNA copies per reaction) were subsequently screened with qPCR, and samples with more than 104 HHV-6 DNA copies per reaction were considered to have iciHHV-6. To validate the approach, we tested a subset of samples with ddPCR to confirm iciHHV-6.16
Clinical data and definitions
Demographic, clinical, and laboratory data were collected from clinical records and databases. Patient and HCT characteristics are detailed and defined in Table 1. A comparison of included and excluded patients during the study period is in supplemental Table 1, available on the Blood Web site. Acute GVHD grades and organ-specific types (skin, gastrointestinal, and liver) and stages were categorized as previously described.20 Chronic GVHD was categorized according to National Institutes of Health consensus guidelines.21 CMV reactivation was defined as any level of plasma CMV DNA or pp65 antigenemia. High-level CMV reactivation was defined as more than 1000 CMV DNA copies/mL plasma or more than 10 pp65 antigen-positive cells per 200 000 peripheral blood leukocytes.22,23 Overall mortality was defined as mortality occurring for any reason. Nonrelapse mortality (NRM) was defined as mortality occurring for reasons other than relapse in patients receiving myeloablative HCTs or for reasons other than relapse or progression of underlying disease in patients receiving nonmyeloablative HCTs. The definitions of engraftment end points were as follows: platelet engraftment, more than 50 000 platelets/µL without transfusion for 1 week; neutrophil engraftment, more than 500 cells/µL for 3 days; and lymphocyte engraftment, more than 300 cells/µL for 3 days.
Recipient, donor, and HCT characteristics stratified by recipient–donor iciHHV-6 status
| Characteristic . | HCT recipient–donor iciHHV-6 status . | |||
|---|---|---|---|---|
| R−/D− (n=4232) . | R+/D− (n=47) . | R−/D+ (n=27) . | R+/D+ (n=13) . | |
| Age of recipient, median (IQR) | 44 (31-54) | 43 (32-56) | 41 (31-53) | 41 (34-54) |
| Age of donor, median (IQR) | 38 (28-48) | 34 (26-47) | 36 (28-50) | 49 (31-59) |
| Sex of recipient | ||||
| Female | 1738 (41.1) | 14 (29.8) | 13 (48.1) | 6 (46.2) |
| Male | 2494 (58.9) | 33 (70.2) | 14 (51.9) | 7 (53.8) |
| Sex of donor | ||||
| Female | 1910 (45.1) | 23 (48.9) | 9 (33.3) | 8 (61.5) |
| Male | 2318 (54.9) | 24 (51.1) | 18 (66.7) | 5 (38.5) |
| Sex mismatch | 1930 (45.6) | 23 (48.9) | 12 (44.4) | 6 (46.2) |
| Race of recipient | ||||
| White | 3512 (85.0) | 46 (97.9) | 23 (92) | 13 (100) |
| African American | 75 (1.8) | 1 (2.1) | 0 | 0 |
| Other | 546 (13.2) | 0 | 3 (8) | 0 |
| Unknown | 99 | 0 | 2 | 0 |
| Race of donor | ||||
| White | 2235 (84.2) | 26 (92.8) | 11 (100) | 12 (100) |
| African American | 61 (2.3) | 1 (3.6) | 0 | 0 |
| Other | 357 (13.5) | 1 (3.6) | 0 | 0 |
| Unknown | 1579 | 19 | 16 | 1 |
| Underlying disease type of recipient* | ||||
| Acute leukemia | 1689 (39.9) | 27 (57.4) | 12 (44.4) | 3 (23.1) |
| Aplastic anemia | 111 (2.6) | 1 (2.1) | 0 | 1 (7.7) |
| Cancer, nonhematologic | 21 (0.5) | 0 | 0 | 0 |
| Chronic leukemia | 1096 (25.9) | 10 (21.3) | 9 (33.3) | 4 (30.8) |
| Lymphoma, Hodgkin | 98 (2.3) | 0 | 2 (7.4) | 0 |
| Lymphoma, non-Hodgkin | 265 (6.3) | 2 (4.3) | 1 (3.7) | 3 (23.1) |
| MDS | 75 (1.8) | 0 | 0 | 1 (7.7) |
| Multiple myeloma | 215 (5.1) | 2 (4.3) | 1 (3.7) | 0 |
| Noncancer | 662 (15.6) | 5 (10.6) | 2 (7.4) | 1 (7.7) |
| CMV serostatus of recipient | ||||
| Positive | 2289 (54.3) | 20 (42.6) | 17 (63.0) | 8 (61.5) |
| Negative | 1928 (45.7) | 27 (57.4) | 10 (37.0) | 5 (38.5) |
| Unknown | 15 | 0 | 0 | 0 |
| CMV serostatus of donor | ||||
| Positive | 1724 (40.1) | 13 (27.7) | 14 (51.9) | 10 (76.9) |
| Negative | 2501 (59.2) | 34 (72.3) | 13 (48.1) | 3 (23.1) |
| Unknown | 7 | 0 | 0 | 0 |
| Recipient or donor CMV positive | 2820 (66.6) | 27 (57.4) | 22 (81.5) | 10 (76.9) |
| Recipient–donor relation | ||||
| Related | 2017 (47.7) | 21 (44.7) | 7 (25.9) | 12 (92.3) |
| Unrelated | 2215 (52.3) | 26 (55.3) | 20 (74.1) | 1 (7.7) |
| HLA-match† | ||||
| HLA-matched | 3279 (77.5) | 40 (85.1) | 19 (70.4) | 10 (76.9) |
| HLA-mismatched | 953 (22.5) | 7 (14.9) | 8 (29.6) | 3 (23.1) |
| Underlying disease severity‡ | ||||
| More advanced | 1696 (40.1) | 18 (38.3) | 14 (51.9) | 6 (46.2) |
| Less advanced | 2536 (59.9) | 29 (61.7) | 13 (48.1) | 7 (53.8) |
| Conditioning regimen¶ | ||||
| Myeloablative | 2877 (68.0) | 33 (70.2) | 20 (74.1) | 9 (69.2) |
| Nonmyeloablative or reduced intensity | 1355 (32.0) | 14 (29.8) | 7 (25.9) | 4 (30.8) |
| Cell source | ||||
| Peripheral blood | 2133 (50.4) | 26 (55.3) | 11 (40.7) | 5 (38.5) |
| Bone marrow | 2099 (49.6) | 21 (44.7) | 16 (59.3) | 8 (61.5) |
| Year of treatment | ||||
| ≥2000 | 2522 (59.6) | 28 (59.6) | 16 (59.3) | 3 (23.1) |
| <2000 | 1710 (40.4) | 19 (40.4) | 11 (40.7) | 10 (76.9) |
| Characteristic . | HCT recipient–donor iciHHV-6 status . | |||
|---|---|---|---|---|
| R−/D− (n=4232) . | R+/D− (n=47) . | R−/D+ (n=27) . | R+/D+ (n=13) . | |
| Age of recipient, median (IQR) | 44 (31-54) | 43 (32-56) | 41 (31-53) | 41 (34-54) |
| Age of donor, median (IQR) | 38 (28-48) | 34 (26-47) | 36 (28-50) | 49 (31-59) |
| Sex of recipient | ||||
| Female | 1738 (41.1) | 14 (29.8) | 13 (48.1) | 6 (46.2) |
| Male | 2494 (58.9) | 33 (70.2) | 14 (51.9) | 7 (53.8) |
| Sex of donor | ||||
| Female | 1910 (45.1) | 23 (48.9) | 9 (33.3) | 8 (61.5) |
| Male | 2318 (54.9) | 24 (51.1) | 18 (66.7) | 5 (38.5) |
| Sex mismatch | 1930 (45.6) | 23 (48.9) | 12 (44.4) | 6 (46.2) |
| Race of recipient | ||||
| White | 3512 (85.0) | 46 (97.9) | 23 (92) | 13 (100) |
| African American | 75 (1.8) | 1 (2.1) | 0 | 0 |
| Other | 546 (13.2) | 0 | 3 (8) | 0 |
| Unknown | 99 | 0 | 2 | 0 |
| Race of donor | ||||
| White | 2235 (84.2) | 26 (92.8) | 11 (100) | 12 (100) |
| African American | 61 (2.3) | 1 (3.6) | 0 | 0 |
| Other | 357 (13.5) | 1 (3.6) | 0 | 0 |
| Unknown | 1579 | 19 | 16 | 1 |
| Underlying disease type of recipient* | ||||
| Acute leukemia | 1689 (39.9) | 27 (57.4) | 12 (44.4) | 3 (23.1) |
| Aplastic anemia | 111 (2.6) | 1 (2.1) | 0 | 1 (7.7) |
| Cancer, nonhematologic | 21 (0.5) | 0 | 0 | 0 |
| Chronic leukemia | 1096 (25.9) | 10 (21.3) | 9 (33.3) | 4 (30.8) |
| Lymphoma, Hodgkin | 98 (2.3) | 0 | 2 (7.4) | 0 |
| Lymphoma, non-Hodgkin | 265 (6.3) | 2 (4.3) | 1 (3.7) | 3 (23.1) |
| MDS | 75 (1.8) | 0 | 0 | 1 (7.7) |
| Multiple myeloma | 215 (5.1) | 2 (4.3) | 1 (3.7) | 0 |
| Noncancer | 662 (15.6) | 5 (10.6) | 2 (7.4) | 1 (7.7) |
| CMV serostatus of recipient | ||||
| Positive | 2289 (54.3) | 20 (42.6) | 17 (63.0) | 8 (61.5) |
| Negative | 1928 (45.7) | 27 (57.4) | 10 (37.0) | 5 (38.5) |
| Unknown | 15 | 0 | 0 | 0 |
| CMV serostatus of donor | ||||
| Positive | 1724 (40.1) | 13 (27.7) | 14 (51.9) | 10 (76.9) |
| Negative | 2501 (59.2) | 34 (72.3) | 13 (48.1) | 3 (23.1) |
| Unknown | 7 | 0 | 0 | 0 |
| Recipient or donor CMV positive | 2820 (66.6) | 27 (57.4) | 22 (81.5) | 10 (76.9) |
| Recipient–donor relation | ||||
| Related | 2017 (47.7) | 21 (44.7) | 7 (25.9) | 12 (92.3) |
| Unrelated | 2215 (52.3) | 26 (55.3) | 20 (74.1) | 1 (7.7) |
| HLA-match† | ||||
| HLA-matched | 3279 (77.5) | 40 (85.1) | 19 (70.4) | 10 (76.9) |
| HLA-mismatched | 953 (22.5) | 7 (14.9) | 8 (29.6) | 3 (23.1) |
| Underlying disease severity‡ | ||||
| More advanced | 1696 (40.1) | 18 (38.3) | 14 (51.9) | 6 (46.2) |
| Less advanced | 2536 (59.9) | 29 (61.7) | 13 (48.1) | 7 (53.8) |
| Conditioning regimen¶ | ||||
| Myeloablative | 2877 (68.0) | 33 (70.2) | 20 (74.1) | 9 (69.2) |
| Nonmyeloablative or reduced intensity | 1355 (32.0) | 14 (29.8) | 7 (25.9) | 4 (30.8) |
| Cell source | ||||
| Peripheral blood | 2133 (50.4) | 26 (55.3) | 11 (40.7) | 5 (38.5) |
| Bone marrow | 2099 (49.6) | 21 (44.7) | 16 (59.3) | 8 (61.5) |
| Year of treatment | ||||
| ≥2000 | 2522 (59.6) | 28 (59.6) | 16 (59.3) | 3 (23.1) |
| <2000 | 1710 (40.4) | 19 (40.4) | 11 (40.7) | 10 (76.9) |
Data are presented as No. (%) unless otherwise indicated. Percentage was calculated based on the number of known observations.
iciHHV-6, inherited chromosomally integrated HHV-6; IQR, interquartile range.
Non-hematologic cancers included breast cancer, sarcoma, neuroblastoma, medulloblastoma, melanoma, cervical cancer, and renal cell carcinoma. Non-cancer underlying disease included primary immunodeficiencies and other non-malignant hematologic disorders.
HLA-match indicates 10/10 allele or antigen match.
More advanced underlying disease refers to diagnoses other than acute myeloid leukemia, acute lymphoblastic leukemia, or lymphoma in first remission, chronic myeloid leukemia in chronic phase, and refractory anemia without excess blasts.
Myeloablative regimens included: any regimen containing ≥800 cGY TBI, any regimen containing carmustine/etoposide/cytarabine/melphalan (BEAM), or any regimen containing busulfan/cyclophosphamide with or without antithymocyte globulin.
Statistical considerations
We compared baseline characteristics using the 2-sided χ-square test or Fisher exact test, as appropriate. We used McNemar's test to evaluate whether the proportion of iciHHV6-positive individuals was equal between patients and donors in the entire cohort. We used Kaplan-Meier curves to show the post-HCT incidence of acute GVHD, overall mortality, and time to neutrophil, lymphocyte, and platelet engraftment by recipient–donor iciHHV-6 status. We performed univariable and multivariable Cox proportional hazards models to evaluate the association of recipient–donor iciHHV-6 status with the following outcomes: acute GVHD grades 2-4 or grades 3-4, chronic GVHD by day 365 post-HCT, any or high-level CMV viremia by day 100 post-HCT, early (day 100) or late (5-year) overall mortality, and NRM. Additional potential predictors of these outcomes included in the univariate analyses were age, sex (donor and recipient), sex mismatch, CMV serostatus, year of HCT, underlying disease risk, conditioning regimen intensity, HLA matching, donor relation, and cell source. For models with endpoints of GVHD or CMV reactivation, death was treated as a competing risk event. For models with the endpoint of death, acute GVHD was treated as a time-varying risk factor. For NRM, acute GVHD was treated as a time-varying risk factor and relapse was treated as a competing risk event. We further described the frequencies of organ-specific acute GVHD, using the stages that determine acute GVHD grades 2 to 4: stages 3 to 4 skin subtypes and stages 1 to 4 liver and gastrointestinal subtypes. Patients with loss to follow-up were censored. After univariate analyses, variables were considered eligible for multivariable models if the 2-sided P value was <.20. We used backward elimination to select multivariable models. All reported P values are 2-sided and considered significant if P < .05. No adjustments were made for multiple comparisons. The R programming language (version 3.3.1) was used for analyses.
Results
Inherited ciHHV-6 epidemiology in HCT recipients and their donors
We identified 4319 first allogeneic HCT recipients at Fred Hutch who had archived β lymphoblastoid cell lines or PBMCs from both the recipient and donor (8638 individuals) (Table 1). When compared with allogeneic HCT recipients who were not included in the study (supplemental Table 1), included patients’ donors were more likely to be CMV seronegative, unrelated, and HLA-matched. Included patients were also more likely to have less advanced underlying diseases, receive nonmyeloablative or reduced-intensity conditioning regimens, and receive peripheral blood stem cells. There were no cord blood HCT recipients included in the study cohort.
We identified 100 individuals with iciHHV-6 (Table 2). HHV-6B was detected in 71% and HHV-6A in 29% of individuals with iciHHV-6. The distribution of species was similar when stratified by HCT recipient vs donor status. Of the 100 individuals with iciHHV-6, 60 were HCT recipients (60/4319; prevalence, 1.4%), and 40 were donors (40/4319; prevalence, 0.9%). There were 13 HCTs in which both recipient and donor had iciHHV-6 (recipient and donor were related in 12/13 instances). Thus, there were a total of 87 (2%) unique allogeneic HCTs in which the recipient, donor, or both harbored iciHHV-6. The prevalence of iciHHV-6 was significantly higher among HCT recipients than donors (P = .027).
iciHHV-6 screening results
| . | Number (%) . | P value . |
|---|---|---|
| Prevalence of iciHHV-6 | ||
| Entire cohort, recipient–donor pairs* | 4319 | .027 |
| Recipients iciHHV-6+ | 60 (1.4) | |
| Donors iciHHV-6+ | 40 (0.9) | |
| Species distribution of iciHHV-6 | ||
| All iciHHV-6 positive recipients or donors | 100 | |
| HHV-6A | 29 (29) | |
| HHV-6B | 71 (71) | |
| Recipients with iciHHV-6 | 60 | |
| HHV-6A | 15 (25) | |
| HHV-6B | 45 (75) | |
| Donors with iciHHV-6 | 40 | |
| HHV-6A | 14 (35) | |
| HHV-6B | 26 (65) |
| . | Number (%) . | P value . |
|---|---|---|
| Prevalence of iciHHV-6 | ||
| Entire cohort, recipient–donor pairs* | 4319 | .027 |
| Recipients iciHHV-6+ | 60 (1.4) | |
| Donors iciHHV-6+ | 40 (0.9) | |
| Species distribution of iciHHV-6 | ||
| All iciHHV-6 positive recipients or donors | 100 | |
| HHV-6A | 29 (29) | |
| HHV-6B | 71 (71) | |
| Recipients with iciHHV-6 | 60 | |
| HHV-6A | 15 (25) | |
| HHV-6B | 45 (75) | |
| Donors with iciHHV-6 | 40 | |
| HHV-6A | 14 (35) | |
| HHV-6B | 26 (65) |
A total of 8638 individuals were screened, and we identified 100 recipients or donors with iciHHV-6 in this cohort. Both recipient and donor had iciHHV-6 in 13 cases, so there were 87 unique hematopoietic cell transplants involving recipient–donor pairs harboring iciHHV-6.
Most HCT recipient and donor characteristics appeared to be similar when stratified by iciHHV-6 source (Table 1). Among HCT recipients with iciHHV-6 (R+/D− and R+/D+ groups, Table 1), there was no statistically significant difference in the distribution of underlying diseases for which recipients received an allogeneic HCT, including malignant and nonmalignant conditions, compared with recipients without iciHHV-6 (P = .87, Fisher’s exact test). However, the comparisons had limited observations. Among donors with iciHHV-6 (R−/D+ and R+/D+ groups; Table 1), CMV seropositivity was more frequent compared with donors without iciHHV-6 (60.0% vs 40.7%; P = .015, χ-square test).
Inherited ciHHV-6 recipient–donor status and risk for acute or chronic GVHD
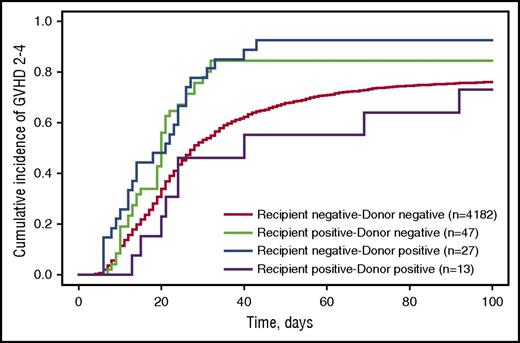
Fifty HCT recipients had unknown acute GVHD status and were excluded from these analyses; none had iciHHV-6. Among the remaining 4269 recipients, grade 2-4 acute GVHD was diagnosed in 3089 (73.9%) recipient–donor pairs without iciHHV-6, 39 (83.0%) recipient positive–donor negative pairs, 25 (92.6%) recipient negative–donor positive pairs, and 9 (69.2%) dual-positive pairs. There was a higher cumulative incidence of acute GVHD grades 2-4 when recipients or donors had iciHHV-6 (Figure 1). In multivariable Cox proportional hazards models, iciHHV-6 in recipients or donors was significantly associated with acute GVHD grades 2-4 (Table 3). The risk was similar for iciHHV-6 recipient positive–donor negative and recipient negative–donor positive HCTs (adjusted hazard ratio [aHR], 1.7 and 1.9; P = .004 and < .001, respectively). Patients in whom both recipient and donor harbored iciHHV-6 were not found to be at increased risk compared with patients without any iciHHV-6; however, this group was small and had a high proportion of related donor HCTs. Whether mismatch in iciHHV-6 status has differential risk compared with any iciHHV-6 is unclear. There was also an apparent increased risk for acute GVHD grades 3-4 among iciHHV-6 recipient negative–donor positive HCTs, but the difference was not statistically significant (aHR, 1.8; 95% CI, 0.9-3.4; P = .10; supplemental Figure 1).
Cumulative incidence probability estimates for acute GVHD grades 2-4 stratified by recipient–donor iciHHV-6 status. The cumulative incidence of developing acute GVHD grades 2-4 by day 100 postallogeneic HCT. Death was treated as a competing risk event. Fifty patients were missing acute GVHD data and were excluded from these analyses.
Cumulative incidence probability estimates for acute GVHD grades 2-4 stratified by recipient–donor iciHHV-6 status. The cumulative incidence of developing acute GVHD grades 2-4 by day 100 postallogeneic HCT. Death was treated as a competing risk event. Fifty patients were missing acute GVHD data and were excluded from these analyses.
Multivariable Cox models for endpoints stratified by iciHHV-6 status
| Endpoint . | HCT recipient–donor iciHHV-6 status . | |||
|---|---|---|---|---|
| R−/D− (n=4232) . | R+/D− (n=47) . | R−/D+ (n=27) . | R+/D+ (n=13) . | |
| Acute GVHD, grades 2-4* | Ref | 1.7 (1.2-2.3)† | 1.9 (1.3-2.8)‡ | 0.9 (0.5-1.7) |
| Acute GVHD, grades 3-4¶ | Ref | 0.7 (0.3-1.4) | 1.8 (0.9-3.4) | 0.6 (0.2-2.5) |
| Chronic GVHD, 1 y§ | Ref | 1.0 (0.7-1.6) | 1.4 (0.9-2.2) | 2.1 (1.0-4.5) |
| CMV viremia, any‖ | Ref | 1.7 (1.1-2.6)** | 1.4 (0.8-2.3) | 1.0 (0.5-2.2) |
| CMV viremia, high-level†† | Ref | 3.1 (1.7-5.8)‡ | 1.9 (0.9-4.0) | 0.5 (0.07-3.7) |
| Overall mortality, 100 d‡‡ | Ref | 1.2 (0.6-2.5) | 0.5 (0.2-1.5) | 1.6 (0.5-4.9) |
| Overall mortality, 5 y¶¶ | Ref | 0.8 (0.5-1.3) | 0.8 (0.4-1.5) | 1.9 (0.8-4.2) |
| NRM, 1 y§§ | Ref | 0.8 (0.4-1.5) | 0.8 (0.4-1.6) | 1.5 (0.6-3.9) |
| Endpoint . | HCT recipient–donor iciHHV-6 status . | |||
|---|---|---|---|---|
| R−/D− (n=4232) . | R+/D− (n=47) . | R−/D+ (n=27) . | R+/D+ (n=13) . | |
| Acute GVHD, grades 2-4* | Ref | 1.7 (1.2-2.3)† | 1.9 (1.3-2.8)‡ | 0.9 (0.5-1.7) |
| Acute GVHD, grades 3-4¶ | Ref | 0.7 (0.3-1.4) | 1.8 (0.9-3.4) | 0.6 (0.2-2.5) |
| Chronic GVHD, 1 y§ | Ref | 1.0 (0.7-1.6) | 1.4 (0.9-2.2) | 2.1 (1.0-4.5) |
| CMV viremia, any‖ | Ref | 1.7 (1.1-2.6)** | 1.4 (0.8-2.3) | 1.0 (0.5-2.2) |
| CMV viremia, high-level†† | Ref | 3.1 (1.7-5.8)‡ | 1.9 (0.9-4.0) | 0.5 (0.07-3.7) |
| Overall mortality, 100 d‡‡ | Ref | 1.2 (0.6-2.5) | 0.5 (0.2-1.5) | 1.6 (0.5-4.9) |
| Overall mortality, 5 y¶¶ | Ref | 0.8 (0.5-1.3) | 0.8 (0.4-1.5) | 1.9 (0.8-4.2) |
| NRM, 1 y§§ | Ref | 0.8 (0.4-1.5) | 0.8 (0.4-1.6) | 1.5 (0.6-3.9) |
Data are presented as adjusted hazard ratio (95% confidence interval).
R+ or R− indicates recipient positive or negative for iciHHV-6; D+ or D−, donor positive or negative for iciHHV-6; GVHD, graft versus host disease.
The R−/D− group excluded 50 patients missing acute GVHD data. Adjusted for conditioning regimen, degree of HLA match, donor relation, and transplant year.
P < .01.
P < .001.
Adjusted for degree of HLA match, donor relation, and transplant year.
Adjusted for age, underling disease risk, conditioning regimen, degree of HLA match, donor relation, conditioning regimen, and transplant year.
Adjusted for age, recipient or donor CMV seropositive, degree of HLA match, and transplant year.
P < .05
Adjusted for age, recipient or donor CMV seropositive, degree of HLA match, transplant year, and acute GVHD grades 3-4.
No individuals were lost to follow up within the first 100 d. Models adjusted for age, recipient or donor CMV seropositive, underlying disease risk, degree of HLA match, donor relation, transplant year and acute GVHD grades 3-4.
Only individuals living past 100 d were included in this model. Adjusted for conditioning regimen, degree of HLA match, donor relation, and transplant year.
Adjusted for age, recipient or donor CMV seropositive, underlying disease risk, degree of HLA match, degree of HLA match, donor relation, transplant year, and acute GVHD grades 3-4.
Among patients with acute GVHD grades 2-4, the distribution of organ-specific subtypes (ie, skin, gastrointestinal, and liver) was not found to differ when stratified by recipient–donor iciHHV-6 status (supplemental Table 2). The small number of observations limited conclusions, and adjusted analyses were not performed. Similar rates of acute GVHD grades 2-4 were seen when stratified by donor relation and HLA match (supplemental Table 3), as well as by iciHHV-6A and iciHHV-6B (data not shown).
There was a marginally increased risk for chronic GVHD within 1 year after HCT among those in whom both recipient and donor harbored iciHHV-6 compared with HCTs in which neither recipient nor donor harbored iciHHV-6 (aHR, 2.1; 95% CI, 1.0-4.5; P = .061; Table 3), but the small size of this group limited definitive conclusions.
Inherited ciHHV-6 recipient–donor status and risk for CMV
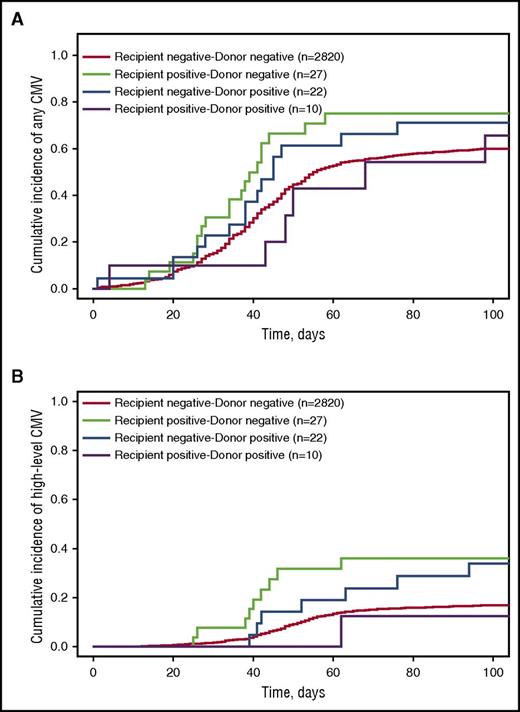
CMV viremia was detected at any level in 1650 (38.7%) patients, and at high level in 460 (10.8%) patients within the first 100 days post-HCT. There was a higher cumulative incidence of any and high-level CMV reactivation after HCT when recipients or donors had iciHHV-6 (Figure 2 and supplemental Table 4). In adjusted Cox models, iciHHV-6 in recipients was associated with increased risk for any (aHR, 1.7; P = .040) and high-level (aHR, 3.1; P = .001) CMV viremia (Table 3). Smaller potential, but nonsignificant, associations with CMV viremia were noted in HCTs in which the donor had iciHHV-6. When stratified by conditioning regimen intensity, increased rates of CMV reactivation among HCTs involving iciHHV-6 appeared to be higher among patients receiving nonmyeloablative conditioning regimens (supplemental Table 4).
Cumulative incidence probability estimates for any and high-level CMV detection stratified by recipient–donor iciHHV-6 status. The cumulative incidence of (A) any or (B) high-level CMV detection (in HCTs with CMV seropositive recipients or donors) by day 100 postallogeneic HCT. High-level CMV reactivation was defined as >1000 CMV DNA copies/mL plasma or >10 pp65 antigen-positive cells per 200 000 peripheral blood leukocytes. Death was treated as a competing risk event.
Cumulative incidence probability estimates for any and high-level CMV detection stratified by recipient–donor iciHHV-6 status. The cumulative incidence of (A) any or (B) high-level CMV detection (in HCTs with CMV seropositive recipients or donors) by day 100 postallogeneic HCT. High-level CMV reactivation was defined as >1000 CMV DNA copies/mL plasma or >10 pp65 antigen-positive cells per 200 000 peripheral blood leukocytes. Death was treated as a competing risk event.
Inherited ciHHV-6 recipient–donor status and risk for overall mortality and NRM
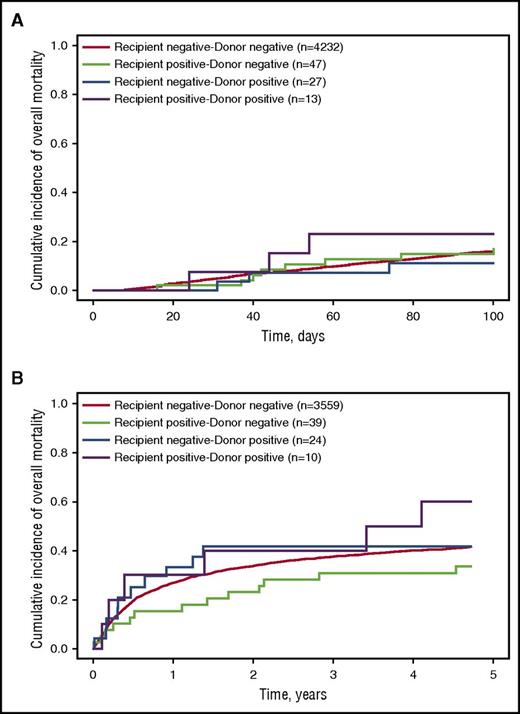
Of the 87 allogeneic HCTs in which the donor and/or recipient had iciHHV-6, 14 (16%) died of any cause by day 100 post-HCT, and an additional 29 (33%) died between day 100 and 5 years post-HCT. There were no significant differences in the proportion of patients who died when stratified by iciHHV-6A or iciHHV-6B (data not shown). Among HCT recipient–donor pairs without iciHHV-6, 673 (16%) died of any cause through day 100 post-HCT, and an additional 1460 (34%) died between day 100 and 5 years post-HCT. Figure 3 shows Kaplan-Meier probability curves of overall mortality by day 100 and 5 years, stratified by recipient–donor iciHHV-6 status. We did not find any associations of early or late overall mortality with recipient–donor iciHHV-6 status in either univariable or multivariable Cox proportional hazards models (Table 3). Using interaction terms, we also examined whether the association of recipient–donor iciHHV6 status and mortality differed by donor relatedness and by degree of HLA match, but these were not influential. Finally, there was no association between iciHHV-6 recipient–donor status and NRM within 1 year of HCT (Table 3).
Kaplan-Meier curves of probability of overall mortality after allogeneic HCT stratified by recipient–donor iciHHV-6 status. Probability of overall mortality (A) within the first 100 days post-HCT and (B) between day 101 and 5 years post-HCT. Within the first 100 days, there were 673 deaths (16%) among recipient negative–donor negative pairs, 8 deaths (17%) among recipient positive–donor negative pairs, 3 deaths (11%) among recipient negative–donor positive pairs, and 3 deaths (23%) among recipient positive–donor positive pairs. Between day 101 and 5 years, there were 1746 deaths (41%) among recipient negative–donor negative pairs, 15 deaths (32%) among recipient positive–donor negative pairs, 12 deaths (44%) among recipient negative–donor positive pairs, and 6 deaths (46%) among recipient positive–donor positive pairs.
Kaplan-Meier curves of probability of overall mortality after allogeneic HCT stratified by recipient–donor iciHHV-6 status. Probability of overall mortality (A) within the first 100 days post-HCT and (B) between day 101 and 5 years post-HCT. Within the first 100 days, there were 673 deaths (16%) among recipient negative–donor negative pairs, 8 deaths (17%) among recipient positive–donor negative pairs, 3 deaths (11%) among recipient negative–donor positive pairs, and 3 deaths (23%) among recipient positive–donor positive pairs. Between day 101 and 5 years, there were 1746 deaths (41%) among recipient negative–donor negative pairs, 15 deaths (32%) among recipient positive–donor negative pairs, 12 deaths (44%) among recipient negative–donor positive pairs, and 6 deaths (46%) among recipient positive–donor positive pairs.
Based on the combined overall mortality rate of 16% by 100 days and 41% by 5 years, we had 80% power to detect HRs of at least 2.8 and 2.1, respectively, comparing iciHHV-6 recipient positive–donor negative pairs with negative–negative pairs; the smaller iciHHV-6 groups required larger HRs (range, 4.1-8.5 and 3.0-7.0, respectively). Given that 24% of the NRM events occurred by 1 year, we had 80% power to detect HRs of 2.5 comparing iciHHV-6 recipient positive–donor negative pairs with negative–negative pairs and HRs of 3.6-8.0 for the smaller iciHHV-6 groups.
Inherited ciHHV-6 recipient–donor status and engraftment
Cumulative incidence curves of time to neutrophil, lymphocyte, and platelet recovery by day 100 post-HCT, stratified by recipient–donor iciHHV-6 status, are depicted in supplemental Figure 2. There were no apparent differences in the time to or cumulative incidence of neutrophil or lymphocyte recovery. HCTs in which the donor stem cells harbored iciHHV-6 had a lower cumulative incidence of platelet recovery, although there were no statistically significant associations between iciHHV-6 status and platelet recovery by day 50 in a univariate Cox model (P values between .41 and .67).
Discussion
We applied an innovative group testing approach to a unique sample biorepository from 8638 HCT recipients and donors to identify individuals with iciHHV-6. We demonstrated iciHHV-6 in 1.4% of HCT recipients and 0.9% of their donors. Inherited ciHHV-6 was associated with increased risk for acute GVHD grades 2-4 and CMV reactivation.
A few studies have reported higher rates of iciHHV-6 among hospitalized patients and those with specific cancers compared with population-based studies,6 but the data are inconsistent and often limited by small numbers. HHV-6 integration into human chromosomes may drive human disease via mechanisms such as chromosomal instability or epigenetic effects.24 Telomeres carrying integrated HHV-6 may be unstable, leading to deletions,25 and telomere length has important implications for chromosomal stability and cell viability.26 Indeed, disruption in telomeres is associated with conditions ranging from pulmonary to hematologic diseases.27,28 The biologic plausibility of a malignancy arising from iciHHV-6 is further supported by the consequences of Marek’s disease virus infection in fowl; this α herpesvirus similarly integrates into telomeres and causes aggressive T-cell lymphomas.29 In our cohort, there was no evidence for increased prevalence of a specific malignant or nonmalignant condition, but the sample size for comparisons was limited. Whether iciHHV-6 can contribute to the development of malignancies will require additional epidemiologic and mechanistic study.
We demonstrated that HCT recipients in whom the donor or recipient harbored iciHHV-6 had an almost 2-fold increased risk of developing acute GVHD grades 2-4. These effect sizes are within the same range as those from recent insights in HLA mismatching at DPB1.30 Whether our findings were driven by any iciHHV-6 in recipient or donor vs mismatch in iciHHV-6 status is unclear, as the group of patients in whom both recipient and donor harbored iciHHV-6 was small. Our data also revealed increased risk for any and high-level CMV reactivation among HCT pairs with iciHHV-6, with adjustment for time-dependent acute GVHD. This increase was more apparent among patients receiving nonmyeloablative HCTs, who retain more of the host immune system, but interpretation of the data is limited by small numbers in this subgroup. Despite the increased rates of acute GVHD and CMV reactivation, we did not detect any significantly increased risk for overall mortality or NRM, but our study was only powered to detect large effect sizes, given limited observations.
Direct or indirect mechanisms underlying these associations remain to be elucidated. Inherited ciHHV-6 can result in viral gene expression and lytic replication,5,7-9 and tissue-level gene expression could promote alloreactivity via resident memory T cells.31 HHV-6B reactivation after HCT (in the absence of iciHHV-6) is independently associated with acute GVHD and CMV reactivation.32 Inherited ciHHV-6 in the recipient and host may also have important immunologic consequences that contribute to our findings. Immune tolerance to HHV-6 proteins among individuals with iciHHV-6 has been proposed, and a study demonstrated lower antibody levels to HHV-6 in this setting compared with patients with exogenous infection.33 However, HHV-6-antigen-specific cytokine responses can be detected in individuals with iciHHV-6, suggesting the condition may not be immunologically silent.34 Our understanding of the immunologic effects of prior herpesvirus infections continues to evolve. A large study investigating the effect of Epstein-Barr virus seropositivity on outcomes in HCT recipients with acute leukemia found an association between donor seropositivity and acute and chronic GVHD they attribute to Epstein-Barr virus-infected donor B-cell activity.35 Our findings of increased risk for acute GVHD and CMV reactivation, regardless of the source of iciHHV-6 (donor or recipient), warrant investigations into the immunologic effects of iciHHV-6 and the relative contribution of reactivation from iciHHV-6 vs noninherited HHV-6B on these outcomes after HCT.
The strength of our study lies in the use of a unique biorepository and well-annotated clinical database to perform a large cohort study of outcomes among HCT recipient–donor pairs with iciHHV-6. This is the largest study of the epidemiology of iciHHV-6 and outcomes after HCT. Yet, some analyses were underpowered because of sample size or the number of observed events. We were unable to determine rates of HHV-6 replication among recipients with or whose donor had iciHHV-6 as a result of the limitations of diagnostic testing, wherein PCR assays for HHV-6 DNA cannot discriminate between the integrated virus and replicating virus.12,13 Molecular tests to identify viral products of gene expression (eg, RNA) or viral culture can be used, but require specialized sample preservation. We note that the overall incidence of grade 2-4 acute GVHD is higher at our center than reported at other centers primarily because of the high sensitivity of our approach for the diagnosis of upper gut GVHD using endoscopy.36 The associations in this study should be evaluated in a larger patient cohort from more than 1 center, and further study of potential mechanistic underpinnings will be important.
In conclusion, we demonstrated that iciHHV-6 might result in multiple complications for affected individuals through a potential increased risk for hematologic diseases and higher rates of acute GVHD and CMV reactivation after HCT. Screening for iciHHV-6, with efficient pooling strategies such as implemented in this study, could be considered to guide donor selection, risk stratification, and treatment strategies post-HCT.
Presented as an oral abstract at the 2017 American Society of Blood and Marrow Transplantation Tandem Meeting, Orlando, FL, 23 February 2017 and the 2016 IDWeek meeting, New Orleans, LA, 27 October 2016.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Zach Stednick for help with data collection and management and the Research Cell Bank at Fred Hutch for providing samples. The authors would also like to acknowledge Jon Guan and Michelle Cho for helping with laboratory work.
This work was supported by a pilot grant from the HHV-6 Foundation (J. A. Hill), a New Investigator award from the American Society for Blood and Marrow Transplantation (J. A. Hill), and by National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases grant 5K23AI119133-02; NIH, National Heart, Lung, and Blood Institute grants K24HL093294, HL093294, and P01HL122173; and NIH, National Cancer Institute grants P01CA018029 and P01CA078902.
Authorship
Contribution: J. A. Hill and M.B. were responsible for the design of the study; J. A. Hill, A.S.M., D.M.Z., and M.B. interpreted the data; J. A. Hill, A.S.M., and A.M. analyzed the data and created the figures; data were collected by J. A. Hill and B.M.S.; samples were provided by J. A. Hansen; laboratory work was carried out or supervised by R.H.-S., M.-L.H., and K.R.J.; the paper was drafted by J. A. Hill with input from all other authors; and all authors reviewed and approved the final version.
Conflict-of-interest disclosure: J. A. Hill and D.M.Z. report grant support from Chimerix, Inc. outside the submitted work. M.B. reports grants and personal fees from Merck and Co., Astellas, Shire, Roche/Genentech, Gilead, and Chimerix and personal fees from Clinigen and Microbiotix outside the submitted work. B.M.S. reports proprietary interest with Gilead outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Joshua A. Hill, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mail stop E4-100, Seattle, WA 98109; e-mail: jahill3@fredhutch.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal