Abstract
Introduction
Nephrotic syndrome (NS) is characterized by massive proteinuria (secondary to podocyte injury), hypoalbuminemia, and edema. Increasing evidence suggests that myriad abnormalities of the hemostatic system may be implicated in the pathogenesis of thrombosis during NS. Indeed, thromboelastometry studies by our group and others have suggested a resistance to fibrinolysis in animal models of NS. Research on NS-associated fibrinolytic effects in human patients is scarce however and complicated by conflicting results and limited mechanistic investigations. The aim of the present study was to delineate the relationship between disease severity (proteinuria and hypoalbuminemia) and fibrinolysis using two rodent models of NS and a cohort of human NS patients. We hypothesized that hypofibrinolysis is directly proportional to NS disease severity.
Methods
Using two well established rat models of NS, transgenic diphtheria toxin receptor (DTR) and puromycin aminonucleoside (PAN), we compared markers fibrinolysis to disease severity. A range of severity was induced by a single I.P. injection of diphtheria toxin (0-75 ng/kg IP) or PAN (0-150 mg/kg IV). On day 10 post-injection, morning spot urines were collected and analyzed for protein:creatinine ratio (uPr:Cr). Rats were then anesthetized and venous blood (IVC) was collected into 0.32% NaCitrate/1.45 µM Corn Trypsin Inhibitor and immediately analyzed with ROTEM before being spun down to platelet poor plasma (PPP). Samples were also collected from a local cohort of pediatric and adult NS patients (n=23), along with the corresponding clinical lab data for each patient. Plasma clot lysis assay (CLA) was performed using urokinase (50 IU). Plasma clot turbidity was measured by inducing clot formation with recalified PPP + 0.5 pM TF + 4uM phospholipids, and monitoring absorbance at 405nm. Fibrinolytic markers in plasma were measured by ELISA. Plasmin generation (PG) has not previously been studied in rats, however our initial results suggested that PG is substantially prolonged in rats compared to humans. Thus, through a series of experiments we first developed a PGA protocol optimized for use in rats. Final PG results were obtained over 12 hours using an MCA-fluorogenic substrate with addition of 10 nM tissue plasminogen activator (tPA).
Results
Hypofibrinolysis: The amount of lysis at 60 min (LI60) on ROTEM was significantly correlated with proteinuria in both the DTR and PAN models (R2=0.167; P=0.015 & R2=0.740; P<0.001, respectively). The addition of tranexamic acid to the ROTEM assay (1mg/100ul), abolished lysis (increased the LI60 to 100%) in both healthy and NS rats (~8% & 3.5% abolishment, respectively), suggesting that the increased clot stability is indeed a fibrinolytic defect. These findings were confirmed with the CLA, such that plasma clot lysis was significantly negatively correlated with proteinuria (R2=0.196; P=0.007 & R2=0.214; P=0.010) and significantly positively correlated with hypoalbuminemia (R2=0.310; P<0.001 & R2=0.240; P=0.006), in the DTR & PAN models, respectively. Additionally, plasma clot lysis by CLA was decreased in NS patients with uPrCr ≥2 (n=16) vs. <2 mg/mg (n=7) (96.1 vs 55.2 %, respectively; P=0.041).
Mechanisms of Hypofibrinolysis: In the DTR rats, disease severity was positively correlated with plasma concentrations of Fibrinogen, uPA, PAI-1, and plasma turbidity at baseline and following clot formation, suggesting a difference in clot structure related to disease state.
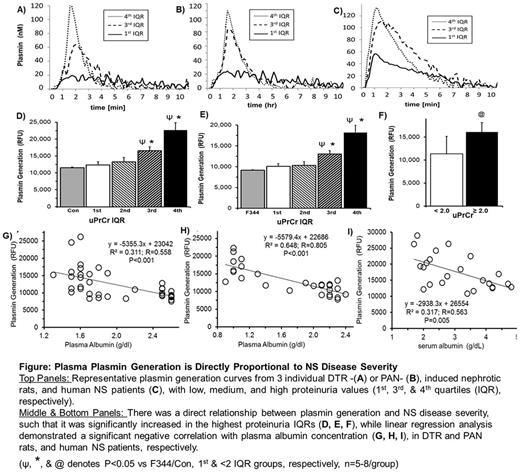
Plasmin Generation: After optimizing the protocol for rats, we were able to obtain evaluable PG curves (Figure). PG increased with disease severity such that it was highly correlated with proteinuria and hypoalbuminemia in both rat models (P<0.001; Figure), as well as in our human samples (P=0.005).
Conclusions
These data suggest that NS thrombi are resistant to fibrinolysis in a manner that is proportional to disease severity. Paradoxically, increased plasmin generation implies that the fibrinolytic system is hyperactive during NS. Thus it appears that the nephrotic clots are inherently resistant to fibrinolysis, even in the face of the increased plasmin that is generated. We are currently examining fibrin clot structure in NS, hypothesizing that nephrotic plasma forms a denser fibrin network that is resistant to fibrinolysis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal