Abstract
Background: The humoral immunogenicity of CAR19, a chimeric antigen receptor with a murine scFV domain developed for treatment with CTL019 (tisagenlecleucel) in relapsed/refractory (R/R) pediatric and young adult acute lymphoblastic leukemia (ALL), was evaluated in 2 studies (ELIANA [NCT02435849]; ENSIGN [NCT02228096]). To date, little is known about the presence and impact of preexisting or treatment-induced anti-murine (m)CAR19 antibodies in patients treated with CAR T-cell therapy.
Methods: Anti-mCAR19 antibodies were measured in patients from ELIANA and ENSIGN trials before and after CTL019 infusion to evaluate the impact of anti-mCAR19 antibodies on cellular kinetics, efficacy, and safety. Data were pooled from ELIANA (n = 68) and ENSIGN (n = 29). Anti-mCAR19 antibodies were captured by mCAR19-transfected Jurkat cells and wild-type Jurkat cells. Following incubation and washing, an R-phycoerythrin (PE)-labeled anti-IgG/M F(ab')2 fragment was added for detection of anti-mCAR19 antibodies using flow cytometry and reported as median fluorescence intensity (MFI). Exploratory assay development included assessment of whether the presence of immunoglobulins after IVIG therapy in clinical samples would interfere with the anti-mCAR19 antibody assay. Impact of preexisting immunogenicity, as detected by MFI levels, was evaluated using cellular kinetic, efficacy, and safety endpoints. The impact of treatment-induced immunogenicity (defined as having an increase in anti-mCAR19 antibody levels over baseline) was assessed by calculating the fold change between preexisting (ie, baseline) levels and postinfusion levels.
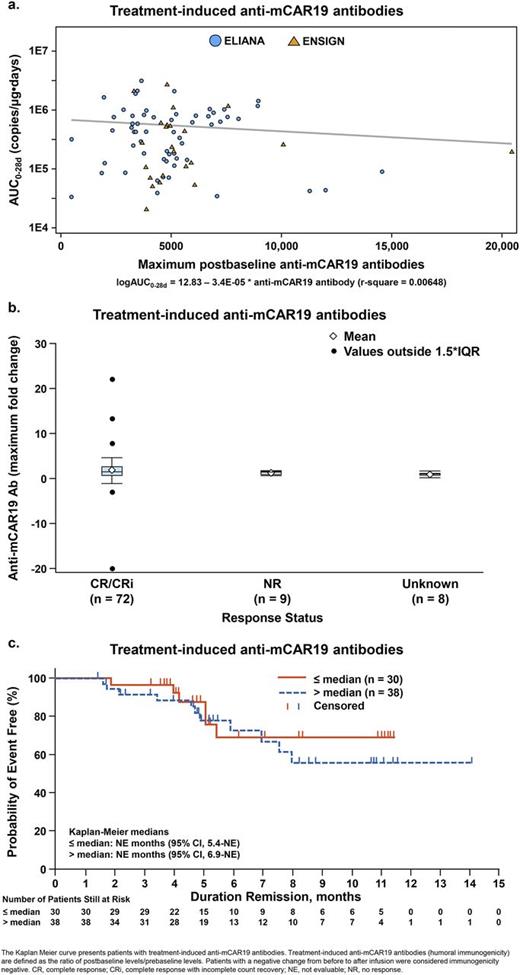
Results: Approximately 90% of patients displayed preexisting anti-mCAR19 antibodies; a similar incidence was detected in healthy volunteer samples, as observed during assay development. Approximately 35% of patients developed treatment-induced anti-mCAR19 antibodies. No apparent relationship between CTL019 expansion (AUC0-28d) and preexisting or treatment-induced anti-mCAR19 antibodies was identified (r2 < 0.001 and r2 = 0.006, respectively); similar results were seen for Cmax (Figure 1a). Presence of anti-mCAR19 after infusion did not appear to influence the persistence of transgene or day 28 response (Figure 1b). Kaplan-Meier curves showed that the presence of preexisting and treatment-induced anti-mCAR19 antibodies did not appear to impact the duration of response or event-free survival (Figure 1c). Strip plots showed consistent levels of preexisting and treatment-induced anti-mCAR19 antibodies across patients with safety events (grade 0/1/2/3/4), including CRS, neutropenia, thrombocytopenia, and neurological events. There was no apparent relationship between treatment- induced anti-mCAR19 antibodies with B-cell recovery categories (recovery ≤ 3 months, > 3 months and ≤ 6 months, > 6 months, and ongoing sustained B-cell aplasia). There was also no association in patients at the time of B-cell recovery with the presence of treatment-induced anti-mCAR19 antibodies following CTL019 infusion. B-cell aplasia requiring immunoglobulin replacement therapy (IVIG) occurred following CTL019 in the majority of patients. The CTL019 concentration time profiles for patients with treatment-induced anti-mCAR19 antibodies were categorized by the time following IVIG administration (within 1 week, 2 weeks, or > 3 weeks of IVIG administration). The results revealed no impact of the time at which IVIG was administered on the in vivo expansion and persistence of the transgene.
Conclusions: We report the first comprehensive assessment of the impact of anti-mCAR antibodies on clinically relevant endpoints for CAR therapy. Determination of immunogenicity is an important endpoint required by health authorities for biologic drugs, and these analyses demonstrate the importance of characterizing the impact on clinical endpoints for CAR therapies. These analyses in patients with pediatric R/R ALL revealed a surprisingly high frequency of baseline anti-mCAR19 antibodies and showed that the presence of preexisting and treatment-induced anti-mCAR19 antibodies did not impact the cellular kinetics, safety, and efficacy of CTL019. Cell-mediated immunity studies are ongoing.
Mueller: Novartis Pharmaceuticals Corporation: Employment. Waldron: Novartis Pharmaceuticals Corporation: Employment. Sickert: Novartis Institutes for BioMedical Research: Employment. Grupp: University of Pennsylvania: Patents & Royalties; Novartis Pharmaceuticals Corporation: Consultancy, Other: grant; Adaptimmune: Consultancy; Jazz Pharmaceuticals: Consultancy. Maude: Novartis Pharmaceuticals: Consultancy, Other: Medical Advisory Boards. Levine: Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees. Laetsch: Novartis Pharmaceuticals Corporation: Consultancy. Pulsipher: Novartis Pharmaceuticals Corporation: Consultancy, Other: Phase II steering committee; Chimerix: Other: Advisory board Dec 2015; Jazz Pharmaceuticals: Other: advisory board, education; CSL Behring: Other: advisory board Feb 2017; Adaptive: Other: Advisory board 6/17. Boyer: Novartis Pharmaceuticals Corporation: Honoraria. August: Novartis Pharmaceuticals Corporation: Consultancy. Awasthi: Novartis Pharmaceuticals Corporation: Employment. Levine: GE Healthcare: Consultancy; Novartis Pharmaceuticals Corporation: Patents & Royalties, Research Funding; Brammer Bio: Consultancy; Tmunity Therapeutics: Equity Ownership, Research Funding. June: WIRB/Copernicus Group: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celldex: Honoraria, Membership on an entity's Board of Directors or advisory committees; Immune Design: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Novartis: Patents & Royalties, Research Funding; Tmunity Therapeutics: Equity Ownership, Research Funding. Taran: Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Wood: Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Myers: Novartis Pharmaceuticals Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal