Abstract
Background: While the mantle cell lymphoma (MCL) international prognostic index (MIPI) and Ki67 are currently utilized to predict outcomes in MCL, cytogenetic abnormalities such as loss(17p) (deletion TP53) and the presence of a complex karyotype (CK) have also been implicated as markers of high-risk disease. Additionally, while WBC count is considered a high-risk feature when calculating MIPI score, a subset of patients with a leukemic, non-nodal presentation can have a very indolent course, suggesting two different populations of patients with leukocytosis. We reviewed the relative impact of a CK as well as individual cytogenetic abnormalities on progression-free (PFS) and overall survival (OS) and utilized these findings to explore the association of WBC count with outcome in MCL.
Methods: We included MCL patients diagnosed between 1993 and 2015 from 5 US-academic institutions. We defined complex karyotype (CK) as > 3 cytogenetic abnormalities in addition to t(11;14), and we collaborated with a clinical cytogeneticist to identify specific abnormalities reported on cytogenetics reports from each patient. We conducted univariable (UV) and multivariable analysis of the 11 most common cytogenetic abnormalities to investigate any association with presence of CK, PFS and OS. Survival distributions were estimated using the Kaplan-Meier method and were compared using log-rank tests. Multivariable (MV) Cox proportional hazards models were fit using backwards elimination method. We then explored the interaction between WBC count and cytogenetic abnormalities with regards to its impact on PFS and OS.
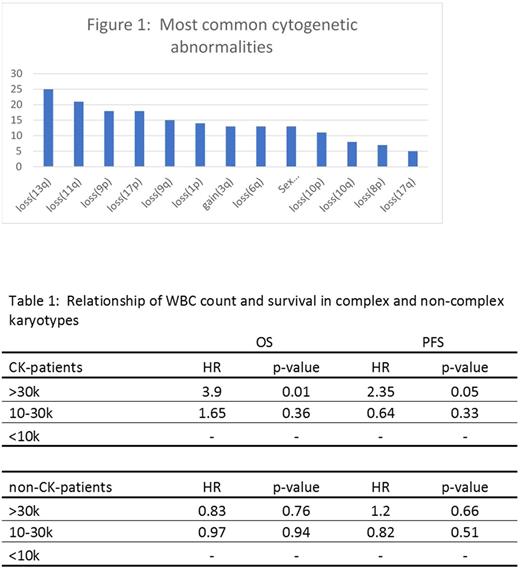
Results: From 277 included patients, there were 117 distinct cytogenetic abnormalities. The most common abnormalities are listed in figure 1. Most patients (69%) were male, 239 (89%) had stage IV disease, and the median age was 63 years (range: 32-83). Fifty-six patients (20%) had a CK. Among patients with available pre-treatment MIPI risk score (n=170); 29%, 39%, and 31% were low-, intermediate-, and high-risk, respectively. CK remained significantly associated with PFS and OS in a MV analysis that contained each of the most common 11 abnormalities (HR 2.03, p=0.003 and HR 2.12, p<0.006 respectively), while none of the individual abnormalities were independently associated with PFS or OS. Loss of 17p was found to be associated with inferior PFS (HR 2.31, p=0.023), but not OS in univariable analysis. In multivariable analysis, neither loss(17p) nor any other specific cytogenetic abnormalities were found to be associated with PFS/OS. None of the 117 distinct cytogenetic abnormalities involved chromosome 2p (site of SOX11).
We then reviewed the impact of CK on the prognostic value of leukocytosis (tables 1 and 2). Patients with an elevated WBC count were more likely to have CK (41% of patients with WBC > 30 had CK compared to 14% of patients with WBC < 10, p=0.002). Among patients with CK, WBC > 30 was associated with inferior OS (HR 3.9, p=0.01) and PFS (HR 2.4, p=0.05). For those without CK, WBC > 30 was not associated with inferior OS (HR 0.83, p=0.76) or PFS (HR 1.2, p=0.66). The interaction between WBC count and CK persisted when WBC was analyzed as a continuous variable as well.
Conclusions: CK remains as an independent predictor of inferior PFS and OS in MCL. Multivariable analysis failed to identify a specific cytogenetic abnormality driving the association of CK with inferior survival. Notably, our analysis did not show an independent association of loss(17p) with survival. In addition, WBC count remains a predictor of poor outcome in MCL patients with CK and does not appear to be associated with indolent disease. MCL patients with CK should be considered high-risk regardless of any specific cytogenetic abnormalities and those with elevated WBC count do not appear to have an indolent course and should be managed aggressively.
Park: Gilead: Speakers Bureau; Takeda: Research Funding; Seattle Genetics: Research Funding; Teva: Consultancy, Research Funding; Cornerstone Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Fenske: Sanofi: Consultancy; Celgene: Consultancy; Pharmacyclics: Consultancy. Hamadani: Sanofi Genzyme: Research Funding, Speakers Bureau; Celgene, Cellerant, Jansen, MedImmune: Consultancy; Takeda, Otsuka, MedImmune, Merck, ADC Therapeutics: Research Funding. Flowers: Burroughs Welcome Fund: Research Funding; Millennium/Takeda: Research Funding; Acerta: Research Funding; Janssen Pharmaceutical: Research Funding; Seattle Genetics: Consultancy; National Institutes Of Health: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Onyx: Research Funding; National Cancer Institute: Research Funding; Infinity: Research Funding; Research to Practice: Research Funding; OptumRx: Consultancy; TG Therapeutics: Research Funding; Genentech/Roche: Consultancy, Research Funding; Spectrum: Consultancy; Bayer: Consultancy; Clinical Care Options: Research Funding; Prime Oncology: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Educational Concepts: Research Funding; Abbvie: Consultancy, Research Funding; V Foundation: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Consultancy. Cohen: Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Research Funding; LAM Therapeutics, Inc: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takada: Research Funding; Bioinvent: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal