Abstract
The availability of imatinib (IM) and thereafter of second-generation tyrosine kinase inhibitors (TKI2) has extended therapeutic options for childhood chronic myeloid leukemia (CML) with a favorable influence on disease outcome and overall prognosis. The aim of this report was to evaluate the response to therapy and long-term outcome of children and adolescents with CML in chronic phase (CP) treated with high-dose imatinib (IM).
CP-CML patients (pts) aged <18 years (yrs) at diagnosis treated with IM at a dosage of 340 mg/m2/day and with a follow-up ≥24 months were considered. Treatment response was evaluate every 3 months by cytogenetics on bone marrow (BM) and/or by quantitative RT-PCR on peripheral blood and/or on BM. A complete cytogenetic response (CCyR) is defined as the absence of Ph+ cells. The terms MR3, MR4, MR4.5 and MR5 are used to indicate BCR-ABL1 IS ≤0.1%, BCR-ABL1 IS ≤0.01%, BCR-ABL1 IS ≤0.0032%, BCR-ABL1 IS ≤0.001%, respectively (Cross et al, 2012). No response, toxicity and loss of response leading to a treatment change were considered as failures.
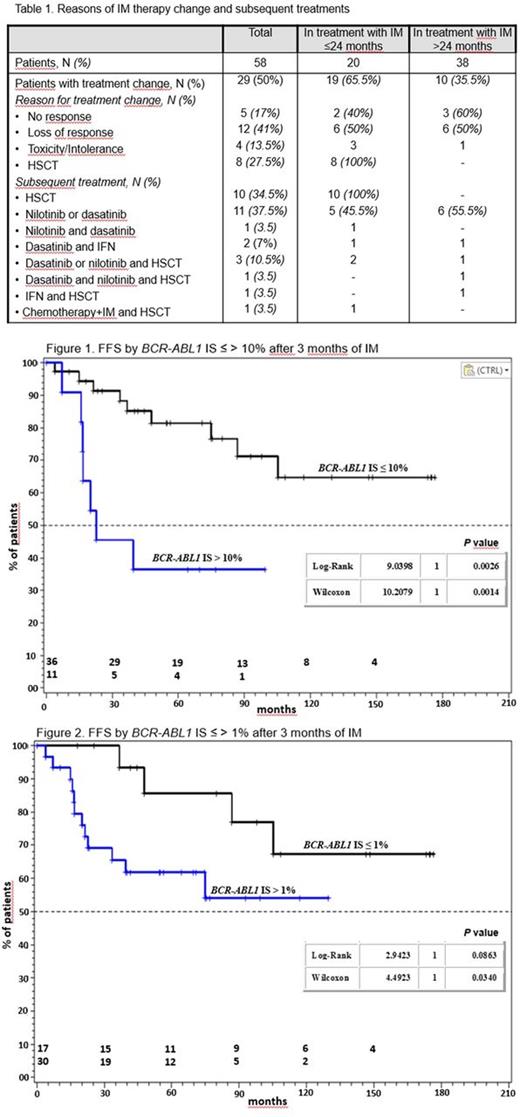
Between March 2001 and June 2015, 58 pts with a diagnosis of CP-CML (males/females 1.6; median age 114/12 years) were treated with IM at dose of 340 mg/m2/day in 14 Italian pediatric centers. Sixteen (27.5%) had an HLA-matched sibling. Thirty-eight pts (65.5%) received IM for a period >24 months. After 3 months of IM treatment, 83% and 56% of ptsshowed BCR-ABL1 IS ≤10% and ≤1%, respectively. BCR-ABL1 transcript levels ≤0.1% were found in 61% and 69% of pts at 12 and 18 months. At 24 months, 66% and 54% of pts showed BCR-ABL1 levels ≤0.1% and ≤0.01%, respectively. Overall, 91%, 85%, 56% and 39% of pts achieved CCyR, MR3, MR4, MR4.5 and MR5 after a median time of 6.1, 12.5, 16.4 and 19 months, respectively. BCR-ABL1 IS ≤10% and ≤1% after 3 months of treatment did not impact on the overall CCyR, MR3, MR4-MR4.5 and MR5. Treatment with IM was stopped in 29/58 pts (50%) after a median time of 16.7 months (range: 1.4-157) because of failure (58.5%), toxicity or intolerance (14%) and allogeneic hematopoietic stem cell transplant (HSCT) (27.5%). Eighteen pts (62%) received one or more TKI2, 3 pts (10%) α-interferon (IFN), 1 pt conventional chemotherapy (3.5%) and 16 pts (55%) underwent a HSCT (Table 1). Transplants were performed after a median of 12 months: 10 from an identical sibling, 5 from a matched unrelated donor and 1 from an umbilical cord blood. Three early transplanted pts had disease recurrence after 24, 36 and 83 months, respectively; they were rescued with IM ± conventional chemotherapy ± 2nd HSCT (2 pts) or dasatinib (1 pt). Among 29 pts who continued IM, 15 (52%) (1 in MR3, 6 in MR4-MR4.5 and 8 in MR5) started intermittent treatment (IM for 3 weeks a month); thereafter, 5 pts resumed continuous IM and 1 patient shifted to dasatinib because of response loss; 3 adolescents with long-lasting BCR-ABL1 IS <0.0032% discontinued treatment. The overall failure-free survival (FFS) at 60 and 120 months was 72.3% (95% CI, 64.1-81.6) and 58.4% (95% CI, 49.8-68.5); transcript levels <10% and <1% BCR-ABL1 ISat 3 months favorably influenced the FFS (Figs 1 and 2). After a median follow-up of 87 months (range: 24-196), 6 pts (10%) are lost to follow-up, 5 (8.5%) have been referred to a Center for adults, 14 (24%) are treatment-free in MR4-MR5(3 pts after 78, 78 and 98 months from IM discontinuation, respectively, and 1 after 20 months from nilotinib discontinuation), 22 (38%) are on IM treatment (1 for a blast crisis after HSCT) and 11 (19%) are receiving a TKI2 ± IFN. All patients are alive, 1 with active disease after transplantation. Two females (1 transplanted and 1 in TKI2) had two pregnancies each (3 healthy children and 1 spontaneous abortion).
In conclusion, this larger cohort of pts with a longer follow-up confirmed our previous results on the high response rates in children and adolescents treated with high-dose IM and the prognostic impact of the transcript levels after 3 months of treatment on the disease outcome. Unfortunately, failures occurred late during treatment with IM. Over time, the efficacy of IM and the availability of TKI2 also in childhood CML has limited the use of HSCT leading to avoid transplants even in children with an identical sibling. Both transplant and TKI did not prevent pregnancies. Although the stop of treatment is not recommended outside of clinical trials, IM or TKI2 can be safely discontinued in some children and adolescents with a durable deep molecular response.
Saglio: Ariad: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Foa: Roche: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Sandoz: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal