Abstract
Graft-versus-host disease (GVHD) is a serious complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Although donor T cells (Teff) have a beneficial effect on graft-versus-leukemia (GVL), they cause mortality and morbidity due to GHVD. Regulatory T cells (Tregs; CD4+Foxp3+) have been demonstrated to efficiently prevent GHVD by suppressing Teff proliferation. In addition to CD4+Foxp3+ Tregs, CD8+Foxp3+induced Tregs (CD8 iTregs) have also been identified not only inhibit Teff function, but also possess the GVL activity. However, instability of CD8 iTregs is an important barrier for GVHD clinical applications. Previous studies have revealed that Janus kinase2 (JAK2) signal transduction is an essential mediator of immune response. JAK2 activation is a positive regulator of Th1 and Th17 differentiation whereas JAK2 acts as a negative regulator in Tregs as its activation leads to Foxp3 instability.
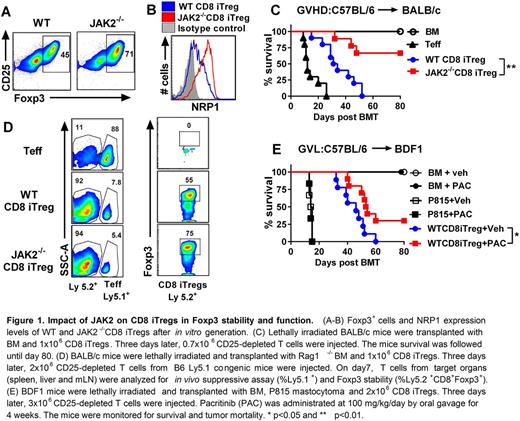
In this current study, we attempted to promote Foxp3 stability in CD8 iTregs by targeting JAK2 kinase using JAK2 conditional knockout mice (JAK2fl/flCD4Cre). The enhanced stability of CD8 iTregs could be a potential Treg-based therapy to prevent GVHD onset while maintaining GVL activity. In the generation of alloreactive iTregs in vitro, we found that a significant higher frequency of Foxp3+ cells could be induced from JAK2-/-CD8 T cells than WT CD8 T cells (Fig. 1A). We also observed that JAK2-/- CD8 iTregs exhibited higher levels of canonical Treg markers, Neuropilin-1 (NRP1), which is known to express on natural Tregs (nTregs) and has been proposed to associate with Treg stability (Fig. 1B). We initially examined in vitro stability of JAK2-/- CD8 iTregs in the presence of proinflammatory cytokines. Strikingly, JAK2-/-CD8 iTregs retained much higher Foxp3 than WT CD8 iTregs. Thus, we further investigated the function of JAK2-/- CD8 iTregs in preventing GVHD after allo-HSCT. Upon iTreg transfer into MHC-mismatched C57BL/6 (B6) to BALB/c model, JAK2-/- CD8 iTregs could significantly alleviate GVHD with a marked reduction of recipient's mortality (Fig. 1C).
To understand the immune mechanisms of JAK2-/- CD8 iTregs in vivo, spleen, liver and mesenteric lymph nodes from recipient mice received JAK2-/- CD8 iTregs were analyzed on day 7 and 14 after allo-HSCT. JAK2-/- CD8 iTregs (Ly5.2+) remarkably suppressed Teff (Ly5.1+) expansion and maintain higher Foxp3 expression compared to WT counterparts (Fig. 1D). Consistently, lower levels of inflammatory cytokines (IFNγ, TNFα, IL-17 and IL-6) were detected from serum of mice received JAK2-/- CD8 iTregs than those of WT iTregs. For translational application, we evaluated the systemic effect of specific JAK2 kinase inhibitor (Pacritinib; PAC) on CD8 iTregs using a GVL model (B6 to BDF1). Mice transplanted with WT CD8iTregs plus P815 mastocytoma and then treated with Pacritinib could significantly prolong the survival and exhibited the GVL activity (Fig. 1E). In conclusion, our studies provide a strong rationale and means to stabilize CD8 iTregs by targeting JAK2 kinase, and stabilizedCD8 iTregs have a therapeutic potential for controlling GVHD in clinic.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal