Abstract
Background: Chimeric antigen receptor transduced T cell (CAR-T) therapy against B-cell malignancies is highly effective, but is frequently associated with cytokine release syndrome (CRS). In severe cases, CRS can be associated with a prolonged life-threatening systemic inflammatory response mimicking manifestations of hemophagocytic lymphohistiocytosis (HLH). Primary HLH occurs among individuals harboring genetic defects in granule-mediated cytotoxicity pathway, such as perforin gene mutations. In a murine primary HLH model, IFNγ derived from antigen-specific CD8+ T cells is implicated as a major mediator of HLH pathology. We explored the role of granule-mediated cytotoxicity and IFNγ in CAR-T response and toxicity, using an established syngeneic murine model of anti-CD19-CAR-T therapy against B-cell malignancy.
Methods: Splenic T cells derived from perforin knockout (Prf-/-), wild-type (WT), and IFNγ adenylate-uridylate-rich element-deleted (ARE-/-: a strain with stable IFNγ mRNA transcript) mice on the B6 background were transduced with second-generation murine anti-CD19 CAR (ScFV-CD28-CD3z). In in vivo experiments, mice received cyclophosphamide (200 mg/kg) intraperitoneally 24 hours prior to CAR-T adoptive transfer as lymphodepletion.
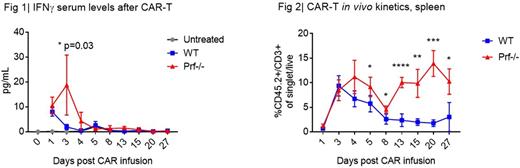
Results: Both WT and Prf-/- T cells were successfully transduced with a mean transduction efficiency of 80%, and resulted in similar immunophenotypic composition (CD8/CD4, TCM/TEM) at baseline. Compared to WT CAR-T, Prf-/- CAR-T produced a higher amount of IFNγ when co-cultured with CD19+ leukemia (12-hour coculture supernatant IFNγ, WT 7822 pg/mL vs. Prf-/- 14743 pg/mL, p<0.0001). In vitro cytotoxicity assays (Incucyte) demonstrated that Prf-/- CAR-T were less efficient in clearing leukemia compared to WT. The differential cytotoxic activity between WT and Prf-/- CAR-T became apparent in vivo when CAR-T were given at low dosage (1E5, which is a curative dosage for WT CAR-T). However, at higher dosage (1E6 or higher), Prf-/- CAR-T eradicated CD19+ leukemia in vivo with the same leukemia clearance kinetics as WT CAR-T and achieved long-term complete remission. During maximal in vivo CAR-T expansion, mice treated with high-dose Prf-/- CAR-T (7E6) had elevated serum IFNγ level, while IFNγ in WT CAR-T group was barely detectable (Fig. 1). Such an increase in IFNγ serum levels did not occur at the lower cell dosage (1E5) in either Prf-/- or WT CAR-T group. Maximal in vivo CAR-T expansion occurred between day 3-4 in both WT and Prf-/- groups. With WT cells, CAR-T contracted by day 8 as leukemia cleared (Fig. 2), and the surface CAR expression dropped and CAR-T phenotype transitioned from TEM to TCM. Surprisingly, Prf-/- CAR-T had a secondary proliferation between day 13-20 (Fig. 2) despite no measurable leukemia or endogenous B-cells at that time. Prf-/- CAR-T had a sustained high level of surface CAR expression and the majority remained in a TEM phenotype. Host-derived CD8+ T-cell phenotype also skewed towards TEM (instead of Tnaive or TCM) in Prf-/- CAR-T group compared to WT CAR-T group. Additionally, spleens derived from Prf-/- CAR-T-treated mice demonstrated increased phagocytosis. These changes in host-derived immune-associated cells suggest that the lack of perforin not only affects CAR-T but may also induce a pro-inflammatory microenvironment through soluble mediators. Among cytokines that we have tested so far (IFNγ, IL-6, TNFα, IL-2, IL-4, IL-17A, IL-10, IL-12p70, MCP-1, GM-CSF), IFNγ has been the only cytokine differentially elevated in vitro and in vivo, comparing Prf-/- to WT CAR-T. IFNγ levels during initial CAR-T expansion phase may be playing a critical role in the persistence of CAR-T. Preliminary results from our ARE-/- CAR-T model also demonstrated that significantly higher serum IFNγ levels during initial CAR-T expansion phase was associated with higher CAR-T in vivo persistence, regardless of antigen availability.
Conclusion: Collectively, these data suggest that perforin is dispensable in murine CD19-CAR-T cytotoxic activity, but contributes to CAR-T contraction, and possibly to the regulation of a secondary inflammatory response. We hypothesize that IFNγ may be the major mediator of the observed differences. Experiments are underway to further elucidate the interplay of perforin and INFγ in CAR-mediated cytotoxicity and to determine whether targeting IFNγ may be a therapeutic option in HLH-like CAR toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal