Abstract
Background
Chronic GVHD (cGVHD) is associated with significant morbidity, decreased quality-of-life, and late mortality after alloHCT. Effective cGVHD prophylaxis is an unmet need. Proteasome inhibitors (PIs) have been shown to suppress dendritic cell (DC) maturation and function and exhibit immunomodulatory effects through inhibition of NF-ΚB, a regulator of cytokine signaling and T and B cell development, activation, differentiation and survival. PIs can deplete proliferating alloreactive T cells, reduces Th1 cytokine production and inhibits APC activation, while sparing regulatory T cells (Tregs). PIs also have inhibitory effects on B and plasma cells, which is important as B cell dysregulation and alloantibody production play a critical role in the cGVHD pathogenesis. In murine and early human studies, PIs have shown efficacy in preventing acute GVHD (aGVHD) and activity in treatment of cGVHD. A novel oral PI, ixazomib (Ixa) has been shown to affect DC maturation and decrease proinflammatory cytokine production and modulate GVHD in a murine model in a schedule-dependent fashion. The safety and efficacy of Ixa after alloHCT in preventing cGVHD is unknown.
Methods
We conducted a single center, open-label, single arm, phase I/II study (NCT02250300) to evaluate the safety and efficacy of Ixa for cGVHD prophylaxis. Adult hematological malignancy patients (pts) undergoing peripheral blood (PB) alloHCT and without active grade (G) 3-4 aGVHD or steroid-refractory aGVHD at enrollment were eligible for the study. Recipients of matched related (MRD) and matched unrelated donor (MUD) alloHCT were enrolled in two parallel and independent cohorts. Alternative donor (cord and haploidentical) and T cell depleted (in vivo or ex vivo) transplants were excluded. Oral Ixa prophylaxis was started between days +60-90 post-alloHCT for a total of 4 weekly doses. In the phase I cohort, two dose levels (DL) were tested in a 3+3 design: 3 mg and 4 mg. Primary outcomes were safety and toxicity profile of Ixa and 1-year cumulative incidence of cGVHD following Ixa administration. The study had 80% power to detect 25% reduction in 1-year cumulative incidence rate of cGVHD diagnosed by NIH Consensus criteria relative toto expected rates in published literature.
Results
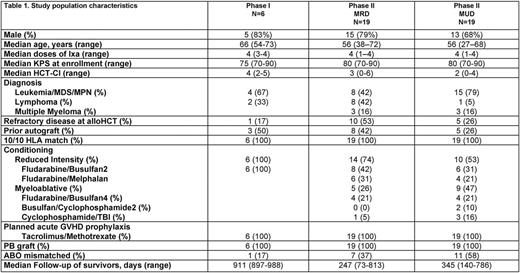
Enrollment started in Jan 2015. Six pts were enrolled on phase I. No dose-limiting toxicities were seen. Ixa was well tolerated; 2 patients had treatment-emergent adverse events on 4 mg DL: one patient had G2 nausea/vomiting (N/V) and G1 diarrhea and other had G1 thrombocytopenia and G2 ALT elevation. The recommended phase II dose was 4 mg weekly. To date, 19 MRD and 19 MUD (target enrollment of 25 and 26 pts, respectively) alloHCT pts have completed treatment on phase II. Table 1 shows baseline patient characteristics. Secondary graft failure and unexpected G≥4 hematologic or non-hematologic toxicities were not seen in phase II portion of the study. Two pts in MRD cohort (N/V=2) and 9 pts (thrombocytopenia=3; N/V=3; relapse=1; others=2) in the MUD cohort missed at least one dose of Ixa. Median follow-up of survivors is 247 days and 345 days for MRD and MUD cohorts, respectively. Median day +100 CD33+ cell chimerism was 100% in both cohorts and median CD3+ cell chimerism was 94% and 97% in MRD and MUD cohorts, respectively. Three patients in MRD cohort and 7 in MUD cohort had active G1/2 aGVHD prior to starting study drug. The cumulative incidence of G2-4 and G3-4 aGVHD at day +180 were 7% (95% CI 5-14%) and 0% in the MRD cohort, respectively. The respective figures for the MUD cohort were 11% (95% CI 4-18%) and 6% (CI 4-11%). Cumulative incidence of any NIH cGVHD at 1 year were 21% (95% CI 9-34%) and 19% (95% CI 9-29%) in the MRD and MUD cohorts, respectively. The respective figures for moderate-severe cGVHD at 1 year were 10% (95% CI 1-19%) and 19% (95% CI 9-29%). One-year non-relapse mortality in the MRD and MUD cohorts was 0% and 5% (95% CI 3-10%), respectively. The cumulative incidence of relapse at 1 year in the MRD and MUD cohorts is estimated at 30% (95% CI 18-42%) and 24% (95% CI 14-34%), respectively. One-year progression-free survival for MRD and MUD cohorts are 70% (95% CI 58-83%) and 71% (95% CI 59-82%), respectively and 1-year overall survival was 100% and 88% (95% CI 80-96%), respectively for the two cohorts.
Conclusion
The early results suggest that Ixa administration is potentially safe and feasible for cGVHD prevention in MRD and MUD alloHCT.
Hari: Novartis: Consultancy; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Honoraria, Research Funding; Takeda Pharmaceuticals: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Spectrum: Consultancy, Research Funding; Janssen: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Shah: Jazz Pharmaceuticals: Consultancy; Geron: Equity Ownership; Exelixis: Equity Ownership; Oncosec: Equity Ownership. D'Souza: Celgene: Consultancy; Prothena: Research Funding; Merck: Research Funding. Dhakal: Celgene: Honoraria. Fenske: Sanofi: Consultancy; Celgene: Consultancy; Pharmacyclics: Consultancy. Hamadani: Celgene, Cellerant, Jansen, MedImmune: Consultancy; Takeda, Otsuka, MedImmune, Merck, ADC Therapeutics: Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal