Abstract
Background:
Hemophilia is an inherited bleeding disorder. Timely and appropriate therapy at time of injury is important to decrease morbidity and mortality. Additionally, the factor concentrates used to treat injuries are expensive and require specialized pharmacy inventory management. In order to have optimal health and economic outcomes, we strove to optimize delivery of appropriate factor doses for injuries in pediatric patients seen in the main campus Emergency Department (ED) via a quality improvement project. Our specific aim was to increase the number of patient encounters between encounters where factor dosing was outside 90-120% of the ideal dose from 4 encounters to 15.
Methods:
In order to establish baseline data, a chart review was completed on all patients with hemophilia A or B seen in the main campus ED for injuries requiring clotting factor concentrate from September 2015 to August 2016. Exclusion criteria included patients with active inhibitors, those on a study concentrate, or those who required a continuous infusion of factor concentrate. The degree of injury described in the medical documentation was reviewed and ideal factor replacement was determined by institutional guidelines. Injuries were classified as minor-requiring a 50% factor correction or major-requiring a 100% factor correction. An ideal dose range in IU/kg was determined for each patient. The ideal dose was then compared to the dose administered in the ED. If factor was given at home prior to the arrival to the ED, and it was documented in the medical record, this was considered in the comparison of dosing. Our pharmacy allows a +/-10% buffer on dosing to permit rounding to appropriate volumes for ease of administration. Taking this hospital standard into consideration, ideal dosing range was defined as 90%-120% of the ideal dosing for the degree of injury. We expanded the upper limit to 120% of the ideal dosing due to the increased risk of bleeding if under dosed, and to also take into consideration the variation in coagulation factor vial sizes. Each month, the ED visits were reviewed and if dosing fell outside the defined ideal range the case was reviewed by the hemophilia clinic director. In an effort to improve efficiency in achieving ideal dosing, we implemented several quality improvement initiatives starting in November 2016. (1) In-service reviews of types of injuries and appropriate dosing were given for hematology/oncology fellows and pharmacists. (2) Treatment dosing algorithms were posted electronically in multiple locations to allow easy access. (3) An electronic medical record based hemophilia injury order set was implemented for ED providers that included links to the treatment guidelines. (4) A departmental communication was sent out to Hematology/Oncology and the ED about the resources available to them. (5) Reminders of changes were integrated into quarterly ED in-services and ED staff meetings. We performed hypothesis testing for categorical variables to evaluate for any other opportunities for specific interventions.
Results:
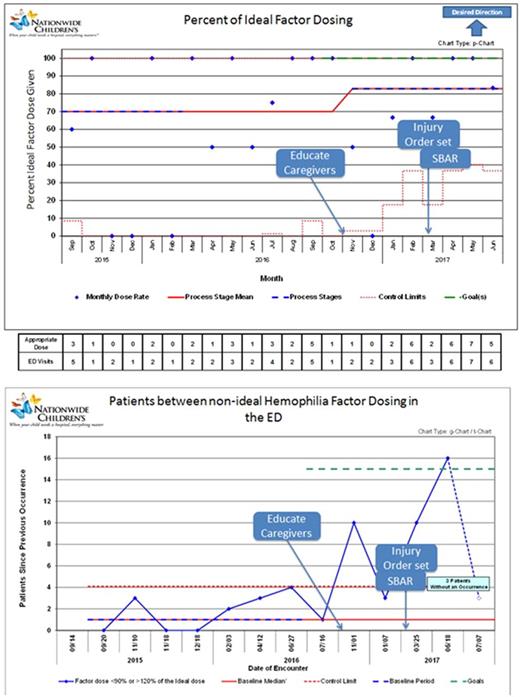
Our baseline data (Sept 2015 - Aug 2016) showed that we were within ideal dosing range 70% of the time. There was no difference between those patients with hemophilia A or B in frequency of out of range dosing ( P =0.15). There was no difference in frequency of out of range dosing between patients treated with standard factor VIII products, standard factor IX products, extended half-life factor VIII or extended half-life factor IX products. We observed no correlation between the hours a pharmacist is in the ED and out of range dosing ( P =0.58). Since the start of our quality improvement intervention in November 2016 to July 2017 we have surpassed our goal and reached 16 encounters between non-ideal dosing, up from our baseline of 4 encounters. We improved our in range average from 70% to 83%.
Conclusion:
Ideal coagulation factor dosing is important for optimal patient care and to ensure economic responsibility. Our quality improvement interventions promoted more accurate dosing of factor for patients with hemophilia seen in the ED for injury related care. Our next steps are to maintain this success over time, and to analyze the financial impact of improved coagulation factor utilization.
Dunn: Alnylam: Other: Unrestricted educational grant; Bayer: Consultancy, Other: Unrestricted educational grant; CSL Behring: Consultancy, Other: Unrestricted educational grant; Biogen: Other: Unrestricted educational grant, Research Funding; Kedrion: Other: Unrestricted educational grant; NovoNordisk: Other: Unrestricted educational grant; Octapharma: Other: Unrestricted educational grant; Shire: Consultancy, Other: Unrestricted educational grant, Research Funding; World Federation of Hemophilia USA: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal