Abstract
Background: Lenalidomide is an immunomodulatory agent FDA approved for the treatment of multiple myeloma (MM) and relapsed/refractory mantle cell lymphoma (MCL). More recently, lenalidomide has been shown to enhance the activity of rituximab and has shown activity in first-line and refractory MCL, diffuse large B-Cell lymphoma (DLBCL), and indolent NHL, both as a single agent and in combination with rituximab. In patients with MM treated with lenalidomide, there is a well-documented risk of venous thromboembolism (VTE), cited as 0.7-0.8 events per 100 patient-cycles. However, this risk has never been well-defined in patients with B-cell NHL treated with lenalidomide and remains an important clinical question. We present preliminary findings of a systematic review and meta-analysis determining rates of venous thrombosis in patients with B-cell NHL treated with lenalidomide. We also present subgroup analyses comparing those patients treated with single-agent lenalidomide and those treated with lenalidomide in combination with chemotherapy or biologic agents.
Aims: Study aims were to assess rates of VTE in B-cell NHL patients treated with lenalidomide
Methods: We conducted a systematic literature search to identify studies in the MEDLINE (1946 to January 2017), EMBASE (1980 to January 2017), and Web of Science Core Collection using the OVID engine, and reviewed the footnotes of all included studies for additional potential studies. We reviewed all abstracts using a structured format and reviewed candidate articles to ensure that they included prospective enrollment of patients with newly diagnosed or relapsed/refractory B-cell NHL, treatment with lenalidomide, and that the number of cycles of lenalidomide given, as well as safety data including rates of VTE, were reported. Two reviewers independently assessed articles for eligibility and extracted data. A VTE event was prospectively defined as Grade II or greater venous thrombosis, and primary outcome defined as VTE events per patient cycle. This was subsequently converted to VTE events per 100 patient cycles for ease of use. The authors were contacted directly if the study would otherwise have met eligibility criteria but did not report all outcomes.
Results: 1719 citations were identified in our initial literature search. Of these, 24 articles were deemed eligible. In total, our review identified 8270 cycles of lenalidomide received by patients with B-cell NHL with 65 VTE events, with an event rate of 0.78 per 100 patient-cycles, a rate very consistent with that of lenalidomide in the treatment of MM.
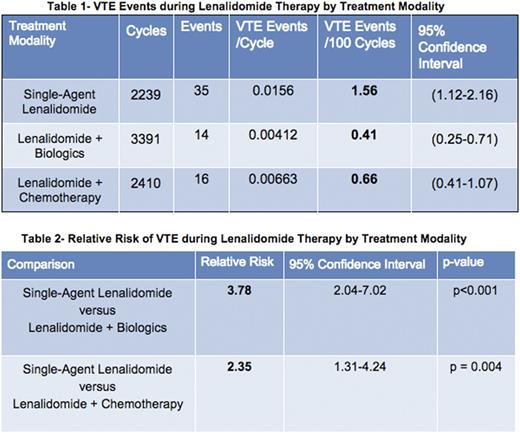
In subgroup analyses, the rate of VTE in patients treated with single-agent lenalidomide was 1.56 per 100 patient/cycles versus 0.41 in patients treated with lenalidomide + biologics with a relative risk (RR) of 3.78 (95% CI: 2.04-7.02, p<0.001). The rate of VTE in patients treated with single-agent lenalidomide was 1.56 per 100 patient/cycles versus 0.66 in patients treated with lenalidomide + chemotherapy with a RR of 2.35 (95% CI: 1.31-4.24, p=0.004). See Tables 1 and 2.
There was no significant difference in rates of VTE between patients treated with lenalidomide + chemotherapy or lenalidomide + biologics. In addition, there was no statistically significant difference between patients treated for DLBCL, MCL, or indolent lymphoma, although our analysis was inadequately powered to detect such a difference. Further risk stratification by disease and patient characteristics, as well as pooled estimates and forest plot creation, is ongoing and will be included in our final report.
Conclusion: Rates of thrombosis in patients with B-cell lymphoma treated with lenalidomide are significantly greater in patients treated with single-agent lenalidomide vs those treated with lenalidomide in combination with either chemotherapy or biologic agents. This is consistent with the findings of a phase II trial by Leonard et al. comparing single-agent lenalidomide and lenalidomide + rituximab in follicular lymphoma that showed a differential risk of thrombosis, although statistical significance was not reached in that study. Rates of thrombosis in patients treated with lenalidomide for B-cell NHL are comparable to rates of VTE in MM. These data inform the consideration of strategies for monitoring and prophylaxis in specific clinical settings.
Leonard: Celgene: Consultancy, Research Funding; Roche: Consultancy. Ruan: Cell Medica: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Consultancy, Research Funding; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal