Abstract
Introduction
Quantitative platelet count is not always a true reflection of an individual's bleeding risk with thrombotic complications previously reported in thrombocytopenic patients. A better laboratory assessment of bleeding risk in these patients is required. Global coagulation assays such as thromboelastography (TEG), thrombin generation with calibrated automated thrombogram (CAT) and fibrin generation assessment with overall haemostatic potential (OHP) assay may provide more complete assessment of an individual's coagulation profile. We aim to evaluate how global coagulation assays differ between thrombocytopenic patients and normal controls.
Methods
Blood samples were collected from thrombocytopenic patients (platelet count <150x109/L). The samples were processed for baseline investigations and global coagulation assays. Citrated whole blood was analysed for thromboelastography using the TEG® 5000 analyser and additional citrated blood samples were double spun at -2500G to obtain platelet-poor plasma (PPP) and stored at -80°C within 2 hours of sample collection. The stored PPP were then used for later testing with CAT and OHP, using standard manufacturer guidelines. The results were compared to our previously collected healthy normal control population (n=90).
Results
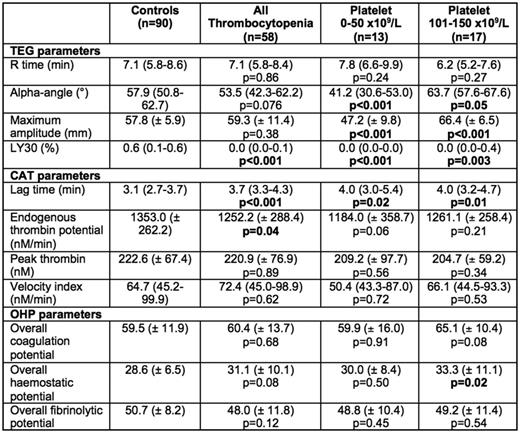
Fifty-eight samples (30 males, 28 females) with mean age of 57.5 years were collected. This included 34 patients with chemotherapy or malignancy-related thrombocytopenia and 24 participants with immune thrombocytopenia or other secondary causes of thrombocytopenia. In comparison to the normal control population (average platelet count 245 x 109/L), thrombocytopenic participants (average platelet count 79 x 109/L, p<0.001) had reduced clot lysis (LY30, 0.0% vs 0.6%; p<0.001) without significant difference in other TEG® parameters including comparable clot strength (maximum amplitude, 59.3 vs 57.8 mm; p=0.38). Thrombin generation using CAT showed reduced endogenous thrombin potential (1252.2 vs 1353.0 nM.min; p=0.040) while fibrin generation showed no difference in the OHP parameters (p>0.05).
On sub-analysis, participants with marked thrombocytopenia (platelet count 0 - 50 x 109/L; n=13) had significantly more hypocoagulable TEG® parameters including prolonged clot formation time (K-time, 3.4 vs 2.3 min; p=0.002) as well as reduced α-angle (41.2° vs 57.9°; p=0.001), clot strength (47.2 vs 57.8 nM.min; p=0.002) and clot lysis (0.0% vs 0.6%; p<0.001). Interestingly, the CAT parameters generally did not meet significance, possibly due to small numbers in this subgroup, apart from prolonged lag time (4.0 vs 3.1 min; p=0.018) although there was a trend towards lower endogenous thrombin potential (1184.0 vs 1353.0; p=0.056). Conversely, participants with platelet count 101 - 150 x 109/L appeared more hypercoagulable on TEG® with shorter clot formation time (1.7 vs 2.3 min; p<0.001) and increased clot strength (66.4 vs 57.8 mm; p<0.001) compared to normal controls. CAT parameters were not significantly different in this subgroup. Fibrin generation was relatively preserved in both sub-groups as well. When comparing the 2 groups of thrombocytopenic patients (ITP vs chemotherapy/malignancy) patients, there was no difference in all the parameters of the three global coagulation assays apart from reduced clot lysis in the latter group (p=0.018)
Conclusion
Global coagulation assays, particularly thromboelastography is useful in measuring the differences in coagulation in thrombocytopenic patients. Marked thrombocytopenia (<50 x 109/L) showed hypocoagulable parameters, which validate current clinical practice of managing this population as high bleeding risk. In contrast, thrombocytopenic patients with platelet counts between 101 - 150 x 109/L were found to be hypercoagulable, providing a possible explanation of overcompensation, which may lead to some thrombocytopenic patients experiencing thrombotic events. Clot lysis was consistently markedly reduced in thrombocytopenic population, suggesting again, possible compensatory mechanisms to reduce bleeding risk. CAT and OHP assay using platelet-poor plasma, on the other hand, does not appear to be sensitive in differentiating the thrombocytopenic and normal healthy populations. Larger prospective studies will be undertaken to confirm these findings.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal