Abstract
Introduction: In AML, IDH mutations can be found at a frequency of 7% for IDH1 and 10% for IDH2 . Precision medicine aims at specifically targeting different gene mutations. Novel drugs which inhibit mutated IDH activity, such as AG-120 (for IDH1), AG-221 (for IDH2) or AG-881 (for IDH1 and 2), are currently tested in clinical trials to assess whether they confer better prognosis and overall survival to patients that carry these mutations.
Aim: To comprehensively investigate the genetics and prognosis of de novo AML with IDH1 R132, IDH2 R140, and IDH2 R172 mutations.
Patients and Methods: In a cohort of 306 de novo AML patients (pts) - all IDH1 or IDH2 mutated - cytomorphology and cytogenetics were available. The cohort comprised 141 females and 165 males, the median age was 65 yrs (range: 21-90 yrs). In all pts a 25-gene panel was investigated by next generation sequencing: ASXL1, BCOR, CALR, CBL, CEBPA, CSNK1A1, DNMT3A, ETV6, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NPM1, NRAS, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, and ZRSR2 . FLT3 -ITD and KMT2A -PTD were analyzed by gene scan. All pts were treated with intensive standard chemotherapy. Overall survival (OS) analyses were performed in comparison to a FAB and MRC (Grimwade et al., Blood 2010;116(3):354-365) matched IDH wild type (wt) reference cohort (n=540).
Results: In total,106 pts (35%) were mutated in IDH1 R132, 166 (54%) in IDH2 R140 and 34 (11%) in IDH2 R172. Regarding amino acidic substitutions, IDH1 R132 was dominated by the R132H substitution in 44 (41%) and the R132C in 39 cases (37%); IDH2 R140 was largely skewed towards the R140Q substitution which occurred in 92% of cases; IDH2 R172 showed R172K in 32 cases (94%) and R172W in 2 cases (6%).
The majority of pts had mutations in two (n=148, 44%) or three (n=78, 23%) of the other 24 analyzed genes (range: 0-6), while only six pts (2%) showed no additional mutations. Among FAB classification, 144 pts (47%) were classified as M1 and 94 (31%) as M2; this rather immature phenotype was most prominent in IDH1 mutated pts with an even higher percentage of M1 (64%) and less of M2 (22%). AML M3 was not present in this cohort at all, indicating a specific occurrence of IDH mutations in distinct AML types. Cytogenetic analyses revealed that 227 pts (74%) had a normal karyotype, 79 (26%) showed an aberrant karyotype with only 6 pts (2%) having a complex karyotype. This is also reflected by a high number of pts in the intermediate MRC group (n=285), while only 6 and 15 pts were grouped to the favorable MRC or adverse MRC group, respectively.
Most frequently IDH mutated pts were also mutated in NPM1 (49%), followed by DNMT3A (40%), SRSF2 (29%), FLT3 -ITD (21%), ASXL1 (16%), NRAS (15%), RUNX1 (15%), and KMT2A -PTD(13%). All other analyzed genes were mutated in <10% of cases. Of note, no IDH2 R172 mutated patient carried a NPM1 mutation, but 63% and 50% of pts mutated in IDH1 R132 and IDH2 R140 (p<0.001). This was also true for NRAS with 18% and 17% of pts mutated in IDH1 R132 and IDH2 R140 (p=0.001). FLT3 was mutated rarely in patients with IDH2 R172 (5%) compared to those with mutations in IDH1 R132 (28%) or IDH2 R140 (32%; p=0.001). In contrast BCOR was often mutated in IDH2 R172 mutated pts (32%) compared to 5% and 7% of pts mutated in IDH1 R132 and IDH2 R140 (p<0.001). SRSF2 was frequently mutated in IDH2 R140 mutated pts (43%; p<0.001) compared to pts mutated in IDH1 R132 (13%) and IDH2 R172 (8%). ASXL1 mutations were rare in IDH1 R132 mutated pts (8%; p=0.007) compared to 22% and 16% in IDH2 R140 and R172 mutated pts.
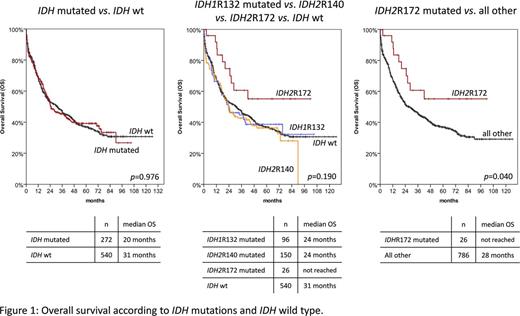
Overall survival analysis did not show significant differences between IDH1 and IDH2 mutated and IDH wt pts. However, when separating IDH1 R132, IDH2 R140, and IDH2 R172 mutated pts, the latter group showed a better prognosis (median OS not reached) compared to all others (median OS: 28 months; p=0.040; Fig. 1). Other worse prognostic factors in IDH mutated pts were higher white blood cell count (WBC) and age, or ASXL1, DNMT3A, SRSF2, and TP53 mutations. NPM1 mutations showed a favorable impact. In multivariate analyses, only IDH R172 mutation, WBC, and age remained independent prognostic factors.
Conclusions: AML pts with IDH1 R132, IDH2 R140, and IDH2 R172 mutations differ in their morphological and genetic patterns. This translates into a more favorable outcome for IDH2 R172 mutated pts. Therefore, IDH mutated AML need further molecular subclassification to define prognosis and select optimal targeted treatment strategies accordingly.
Meggendorfer: MLL Munich Leukemia Laboratory: Employment. Haferlach: MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Kern: MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Haferlach: MLL Munich Leukemia Laboratory: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal