Abstract
Background:
Blast transformed (BT) chronic myelomonocytic leukemia (CMML); defined by ≥20% blood or bone marrow (BM) blasts, occurs in 15-30% of patients and is a major cause of mortality. Reported risk factors include high risk karyotype, circulating blast count, circulating immature myeloid cells (IMC), CMML morphological subtype, and the presence of ASXL1 mutations. In the current study, we reviewed consecutive cases of BT CMML from the Mayo Clinic, MN, and the MD Anderson Cancer Center, Texas, USA, with the intent to examine i) prognostic factors, ii) survival trends, and iii) treatment outcomes.
Methods:
Diagnoses of CMML and BT-CMML were according to the 2016 WHO criteria. Treatment response in BT-CMML patients was assessed according to standard AML response criteria. Statistical analyses considered clinical and laboratory data collected at the time of BT. Survival was calculated from the date of BT to the date of death or last contact.
Results:
171 cases of BT CMML were identified from the Mayo Clinic (n=84) and MDACC (n=87). The median age at BT was 69 years and 64% were male. Eight one (49%) had received prior hypomethylating agent (HMA) therapy (Decitabine 50, 5-azacitidine 31), with 30% having achieved a complete remission (CR) prior to BT. CMML patients that received prior HMA therapy were more likely to have thrombocytopenia (p=0.03), higher LDH levels (p=0.007), lower frequency of TET2 mutations (p=0.04) and higher risk stratification by the Mayo prognostic model (p=0.009). The two institutional cohorts were comparable with regards to variables, both at CMML diagnosis and BT.
Patient characteristics at time of BT: The median time from CMML diagnosis to BT was 12 months (range, 1-36). At BT, 16 (10%) presented with extramedullary disease, 81 (63%) with an abnormal karyotype, 41 (34%) with cytogenetic clonal evolution, with the following mutational distribution; TET2 50%, ASXL1 39%, NRAS 39%, FLT3 ITD 20%, NPM1 6%, RUNX1 6%, and 5% each for IDH1/2, EZH2 and JAK2 . Molecular clonal evolution was seen in 4 (22%) of 18 evaluable cases.
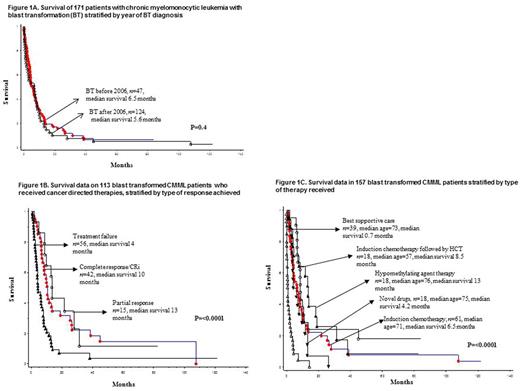
Survival trends: After a median follow-up of 4.4 months (range 0-122), 141 (82%) deaths were recorded. Median overall survival (OS) was 6 months with 1- and 3-year survival rates of 25% and 9%, respectively; survival trends were similar (p=0.4) for patients diagnosed prior to and after the year 2006 (figure 1A).
Treatment and response rates: Specific treatment for BT-CMML was documented in 157 cases and included induction chemotherapy (n=61), induction chemotherapy followed by stem cell transplant (HCT, n=18), HCT alone (n=3), HMA (n=18), novel therapeutic agents (n=18), and best supportive care (BSC, n=39). Treatment response was assessable in 113, including 76 treated with induction chemotherapy, 18 with HMA and 16 with novel therapeutics; with respective CR rates of 24%, 0% and 41%; with an additional 24%, 16% and 16% of patients achieving CR with incomplete blood count recovery (CRi), with a survival similar to those with CR (figure 1B). HCT was reported in 25 patients, 17 myeloablative and 8 reduced intensity, with a median OS of 7 months.
Predictors of survival in BT-CMML: In univariate analysis of variables recorded at time of BT, risk factors adversely impacting survival included older age, lower hemoglobin, PB blast %, adverse cytogenetics at BT, prior exposure to HMA, not receiving induction chemotherapy, not achieving CR or CRi and not receiving HCT; all of these risk factors remained significant when analysis was adjusted for age. In multivariable analysis, lack of prior HMA exposure (p=0.001, HR 5.1; 95% CI 1.9-14.1) and the achievement of CR/CRi (p=0.02, HR 1.4; 95% CI 1.2-3.4) remained significant while HCT lost its significance (p=0.09). Gene mutations, including FLT3 ITD, cytogenetic and molecular clonal evolution at the time of BT did not impact survival. The 3-year survival rates for BT patients receiving induction chemotherapy, induction chemotherapy followed by HCT, HMA, novel agents and BSC were 8%, 20%, 12%, 3% and 0% respectively (figure 1C).
Conclusions: Survival in patients with BT- CMML is dismal (≈6 months) and is adversely impacted by prior exposure to HMA and by the failure to achieve CR/CRi. After BT, the use of HMA therapy is largely ineffective (0% CR), with intensive therapies such as induction chemotherapy with or without HCT, failing to achieve durable remissions.
Jabbour: Amgen: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Takeda Oncology: Research Funding. Kantarjian: Amgen: Research Funding; Delta-Fly Pharma: Research Funding; ARIAD: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Bristol-Meyers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal