Abstract
Introduction
We previously reported the results of the phase III randomized HOVON-87/NMSG-18 study for Newly Diagnosed Multiple Myeloma patients not eligible for stem cell transplantation (nte-NDMM). The efficacy of melphalan, prednisolone and either thalidomide followed by thalidomide maintenance (MPT-T) versus lenalidomide followed by lenalidomide maintenance (MPR-R) was found to be comparable, being consistent across subgroups defined by age, cytogenetic risk and ISS [1]. As frailty is known to affect clinical outcome, we investigated the impact of frailty on outcome.
Methods
Frailty was assessed by a modification of the IMWG frailty score based on age, the Charlson Comorbidity Index (retrospectively retrieved from the list of comorbidities that were present at entry) and the WHO performance as a proxy for (instrumental) Activities of Daily Living ((i)ADL). To assess the effect of frailty on progression free survival (PFS) and OS, the logrank test was used, while the chi-squared test was used to evaluate the association of frailty with discontinuation rate and toxicity.
Results
All 637 eligible patients from the HOVON-87/NMSG-18 trial were included in the analysis. Median age was 73 years; 66% of patients were ≤ 75 years, 24% were 76-80 years and 10% were > 80 years. A CCI of 0, 1, 2 and ≥3 was found in 61, 20, 7 and 4% of patients respectively (8% unknown). The most common comorbidities were diabetes mellitus without end organ complications (12%) and myocardial infarction (6%). A WHO performance of 0, 1 and ≥ 2 was observed in 35, 47 and 16% respectively (3% unknown).
Univariate analyses showed that older age (>80 years: HR 1.59 [95% CI 1.12-2.25]), a higher CCI (CCI ≥2: HR 1.41 [95% CI 1.01-1.95]); and a poor WHO performance (WHO ≥2: HR 2.05 [1.49-2.82]) were associated with an inferior OS. HR's were 1.07 [95% CI 0.82-1.40] and 1.27 [95% CI 0.98-1.65] for age 76-80 years and WHO performance respectively. For age and the CCI the IMWG frailty score classification was used. For the WHO, scores were assigned based on the HR: WHO 0; 0 points, WHO 1; 1 point and WHO ≥2; 2 points. Fit patients were defined as a proxy frailty score of 0, unfit as a score of 1 and frail as a score of ≥2, comparable to the IMWG frailty score.
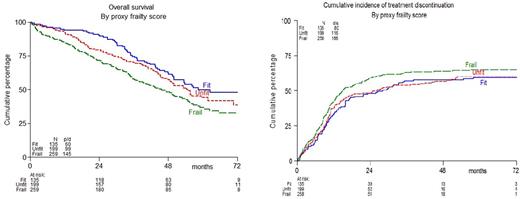
Using this modified IMWG frailty score, 135 patients were fit (21%), 199 were unfit (31%) and 259 were frail (41%) (7% unknown). The median OS was found to be significantly different in fit versus unfit versus frail patients; 58, 55 and 46 months respectively (p=0.004). In contrast no significant difference in median PFS was found; 26, 20 and 21 months respectively (p=0.30).
The inferior OS for the frail might be partly explained by higher discontinuation rate of induction therapy; 50%, versus 43% in unfit, and 34% in fit patients (p=0.011), being mainly due to excessive toxicity (25, 23 and 16% respectively). The cumulative incidence of treatment discontinuation on protocol (induction plus maintenance), corrected for death and progressive disease which were considered as competing risks, is depicted in figure 1B. Discontinuation rate at 2 years for fit, unfit, and frail patients were 48%, 48%, and 59% respectively (p=0.06). There were significantly more grade ≥3 adverse events on protocol in frail and unfit patients (both 86%) as compared to fit patients (77%, p=0.039). Especially more grade ≥3 infections were found in frail (28% versus 18% in unfit and 13% in fit). In contrast, frailty was not associated with hematological toxicity.
Conclusion
We here present a modified frailty score, using the WHO performance instead of the (i)ADL, combined with age and the CCI, that enables the identification of frail MM patients with an inferior OS, a higher discontinuation rate and a higher rate of grade ≥ 3 toxicity. This non-laborious modified frailty score can be easily implemented in clinical trials and allows to compare the outcome of frail patients in nte-NDMM trials, which will hopefully result in frailty-adapted therapy in clinical daily practice.
Reference:
1. Zweegman S, Holt vd B, Mellqvist U, et al. Blood 2016;127(9):1109-16
Mellqvist: Celgene: Honoraria; janssen: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Mundipharma: Honoraria; Sandoz: Other: participation advisory board; Amgen: Honoraria, Other: participation advisory board. Abildgaard: Takeda: Research Funding. Plesner: Janssen: Research Funding; Janssen, Takeda: Consultancy; Janssen, Genmab: Membership on an entity's Board of Directors or advisory committees. Van De Donk: Janssen, Celgene, Bristol-Myers Squibb, Amgen: Research Funding. Sonneveld: Celgene, Amgen, Janssen, Karyopharm, Takeda: Consultancy, Honoraria, Research Funding; Celgene Corporation, Amgen, Janssen, Karyopharm, PharmaMar, SkylineDx: Honoraria; Celgene Corporation, Amgen, Janssen, Karyopharm, SkylineDx, PharmaMar: Consultancy. Waage: Celgene, Takeda: Honoraria. Zweegman: Celgene: Other: advisory board participation, Research Funding; Janssen: Other: advisory board participation, Research Funding; Takeda: Other: advisory board participation, Research Funding; Amgen: Other: advisory board participation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal