Abstract
Introduction: Relapse of the disease remains a major burden after stem cell transplantation (HCT) following myeloablative conditioning and even more after reduced intensity and non-myeloablative conditioning. Efforts to reduce relapse incidence involve early immunosuppression withdrawal and prophylactic buffy coat infusion in high risk patients. In this phase II study we analyzed the role of disease-specific chimerism driven immunosuppression.
Methods: Between May 2008 and July 2012, 200 consecutive patients (pts) (130 male and 70 female) with a median age of 62.0 (range 26.2-74.8) years (y) with hematological malignancies were transplanted after NMA conditioning. The conditioning regimen consisted of fludarabine 30mg/m2 and total body irradiation (200 cGy) followed by immunosuppression with cyclosporine A (CSA) and mycophenolate mofetil (MMF). The primary objective of the study was to determine the incidence of relapse after a decrease of >10% disease-specific donor cell chimerism (DCC). Furthermore, the incidence of graft vs. host disease (GvHD) and the reconversion of DCC were investigated.
Pts were transplanted for acute myeloid leukemia (AML; n=85; 42.5%), multiple myeloma (MM; n=31; 15.5%), myelodysplastic syndrome (MDS; n=26; 13%), chronic lymphocytic leukemia (CLL; n=22; 11%), B-Non-Hodgkin Lymphoma (B-NHL; n=11; 5.5%), T-NHL (n=6; 3.0%), acute lymphoblastic leukemia (ALL; n=7; 3.5%), myeloproliferative neoplasia (MPN; n=6; 3%), MDS/MPN (n=3; 1.5%) and chronic myeloid leukemia (CML; n=4; 2%). Donors were matched-related (MRD)(n=26;13%), matched unrelated (MUD) (n=129; 64.5%) and mismatched unrelated on allele or antigen level (n=45;22.5%).
DCC analyses were performed on flow sorted cells on day (d)+ 28, d +56, d+ 84, d + 180 and every 6 months following. As a disease specific DCC line we used CD34 for AML, MDS and MPN pts, CD19 for CLL and B-NHL, CD138 for myeloma pts. The pts with T-NHL were monitored by CD3 chimerism. In addition, engraftment was monitored by CD3 chimerism.
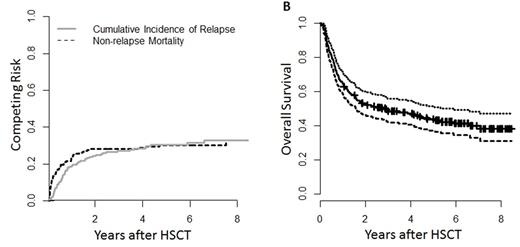
Results: Overall survival was 52.9 (95% Confidence Interval (CI) 46.4 - 60.3)% at 2y, and 43.9 (CI 37.3 - 51.6)% at 5y. Relapse incidence was median 28.2 (CI 22.1 - 34.6)% at 2 y and 30.3 (CI 23.9 - 36.9)% at 5 y. Non-relapse mortality (NRM) was 24.3 (CI 18.5-30.5)% at 2y and 30.4 (CI 23.9-37.1)% at 5y.
DCC (≥90%) was observed in 116 of the 200 (58.0%) pts, 43 patients (21.5%) showed incomplete disease-specific DCC or reduction of DCC in association with hematological relapse: AML (n=17), MDS (n=4), MM (n=7), CLL (n=7), B-NHL (n=1), T-NHL (n=1), MPN (n=2), ALL (n=2), CML (n=1), MDS/MPN (n=1) and 41 patients (20%) had an isolated line specific DCC decrease after a median of 106 (28-2975) days following HCT.
In the 41 pts with decrease in disease-specific DCC without hematological relapse, 1 was off immunosuppression and 3 pts had an active acute or chronic GvHD. In 37 pts CSA and MMF was reduced prematurely. 5 pts showed acute GvHD grade ≥III after reduction and 32 pts GvHD grade 0-II. Of the 37 pts, 27 (73.0%) showed no increase of DCC. 24 (88.9%) pts died, 18 due to relapse, 6 due to non-relapse causes. 3 pts are alive after salvage chemotherapy. 10 of the 37 patients (26.5%) reached full DCC after a median of 29 (21 - 267) days following immunosuppression taper and one recovered from the decrease of DCC and hematological relapse after discontinuing the immunosuppression.
43 pts (15.5%) showed no full DCC with (n=12) or without hematological relapse (n=31). Following reduction of immunosuppression 32 pts relapsed, of which 4 are alive after salvage therapy. 2 pts rejected the HCT and are alive after 2nd HCT.
85 AML pts were separately analysed, of which 43 experienced a complete DCC at any time point (27 are alive without relapse, 16 died due to non-relapse causes). 25 (29.4%) of which 24 pts were without active GvHD had isolated DCC decreased and were tapered. 7 converted to full DCC. All 17 pts showing no full disease specific DCC with or without simultaneous relapse relapsed. 15 pts died of relapse, 2 are alive after salvage therapy.
Conclusion: Decrease of disease-specific DCC was observed in 20% of pts and in 29.4% of the AML pts. Of those 27.7 % in the total population and 29.2 % in the AML group showed recovery of DCC without hematological relapse after tapering of immunosuppression. We conclude that DCC is a useful tool to avoid relapse in the early phase of HCT while the patient is on immunosuppression.
Franke: Novartis: Honoraria, Research Funding; Pfizer: Honoraria; Takeda: Consultancy. Lange: Novartis: Honoraria, Research Funding. Schwind: Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal