Abstract
Introduction
The Choosing Wisely initiative aims to reduce wasteful testing. Our institution is participating in this initiative and hence has a focus on evidence-based approaches to the investigation of FN. We have previously shown that routine chest x-rays are not generally useful in this scenario. Urine cultures are also commonly performed in this context. However, specimen collection may delay the administration of antibiotics, laboratory costs are not inexpensive and the impact of culture results on subsequent antibiotic management is not well documented in patients receiving protocol specified broad spectrum antibiotics. We assessed this latter issue by evaluating the impact of urine cultures on the management of FN in haematology inpatients at a major tertiary hospital.
Methods:
A retrospective single-centre analysis of adult (≥16years) patients with haematological malignancy undergoing recent chemotherapy admitted to a large cancer centre with FN was performed over a 5-year period (2011-2015). FN was defined as fever ≥37.5°C and a neutrophil count <0.5 x 109/L or <1.5 x 109/L and predicted decline to <0.5 x 109/L over the next 48 hours. Local protocolised investigations for newly diagnosed FN included a urine culture. Empiric antibiotic therapy for FN consisted of IV tazocin in stable patients or, if systemically compromised, IV meropenem, gentamicin and vancomycin. Patients were identified from electronic hospital records. For each episode, baseline demographic data, urinary tract symptoms and signs (absence of which was termed 'asymptomatic'), beside urinalysis results (nitrites and leucocytes), urine culture results (chemistry, microscopy, culture and sensitivity) and any consequent change in antibiotic management were collected. A urine culture was considered positive if >108 organisms/L of a predominant organism were detected. Cultures were considered contaminated if mixed growth was present. Each positive culture and impact on antibiotic therapy was reviewed by an infectious disease physician.
Results:
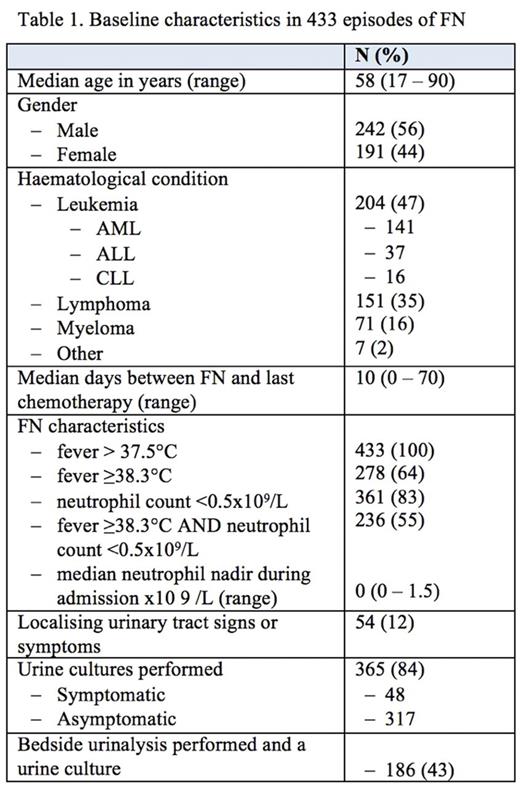
Four hundred and thirty-three episodes of FN were identified in 317 patients, the baseline characteristics of which are summarized in Table 1. A urine culture was performed in 365 (84%) cases of which 16 (4.4%) were positive, 77 were contaminated (21%), and 272 (75%) had no growth. Cultures were positive in 7 of 317 (2.2%) asymptomatic patients; all were gram-negative bacteria of which 2 were extended spectrum beta-lactamase (ESBL) E. coli. Nine of 48 (19%) symptomatic patients had positive cultures of which 8 were non-ESBL E. coli. Four patients had a catheter, all of whom were asymptomatic with a negative culture. Bedside urinalysis was documented in 186 (43) patients who had urine cultures. Nitrites were detected in 5 of 12 evaluable patients with positive cultures compared with 4 of 174 patients with negative cultures; the positive and negative predictive values were 56%and 96%respectively. Only 5 patients (1.4%) had a change in antibiotic management due a positive urine culture: 3 of 48 (6.3%) symptomatic and 2 of 317 (0.6%) asymptomatic patients.
Conclusion:
Urinary tract infections are a rare source of infection in haematology patients with FN in the absence of focal urinary symptoms. Urine cultures rarely impact on antibiotic management in this context without urinary tract symptoms. As a result of this study we no longer perform urine cultures routinely in FN, restricting this investigation to symptomatic or catheterised patients without another clear source of infection, and those with nitrite positivity.
Hawkes: Roche: Other: Travel expenses, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Other: Travel expenses; Celgene: Research Funding; Bristol-Myers Squibb: Research Funding; Roche: Other: Travel expenses; Merck Serono: Research Funding; Merck Sharpe Dohme: Research Funding. Grigg: Merck Sharpe Dohme: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Takeda: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal