Abstract
Introduction: Patients with essential thrombocythemia (ET) are at greater than 20% risk for thrombotic complications. They are categorized into high- and low-risk groups based on the presence of advanced age (> 60 years) and / or thrombosis history. One of the main goals of ET treatment is thrombosis prevention. Use of hydroxyurea (HU) among high-risk ET patients improves rate of thrombosis and is the frontline guideline-recommended therapy for older patients. Little is known about HU impact on the survival and thrombosis in the real-world setting.
Methods: Using the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database, we identified patients diagnosed with ET (International Classification of Diseases for Oncology, 3rd edition, code 9962) from 2007-2011. Patients were required to: 1) be aged 66-99 years; 2) have continuous enrollment in Medicare Parts A and B from one year before diagnosis to death or the end of study (12/31/2012); and 3) have continuous Part D six months before diagnosis to the end of follow-up to capture HU use. HU users were restricted to those who had more than two HU prescription claims. HU coverage was calculated as the percentage of days from diagnosis to the end of follow-up covered by HU prescriptions. Thrombotic events were defined as first occurrence of venous thrombosis, arterial thrombosis, or sudden death after diagnosis. Predictors of receiving HU were evaluated using multivariate logistic regression models. Multivariable Cox proportional hazard regression models were utilized to assess the impact of HU on survival and thrombotic events, controlling for patient age at diagnosis, sex, race, Part D low income subsidy (a proxy marker for lower socioeconomic status), influenza vaccination 12 months prior to diagnosis (an indicator for health care access), disability status (a claims-based proxy for poor performance status), modified Elixhauser comorbidity score (the original comorbidity score excluding cardiovascular conditions), and pre-existing cardiovascular conditions. HU was treated as a time-dependent variable in the Cox model.
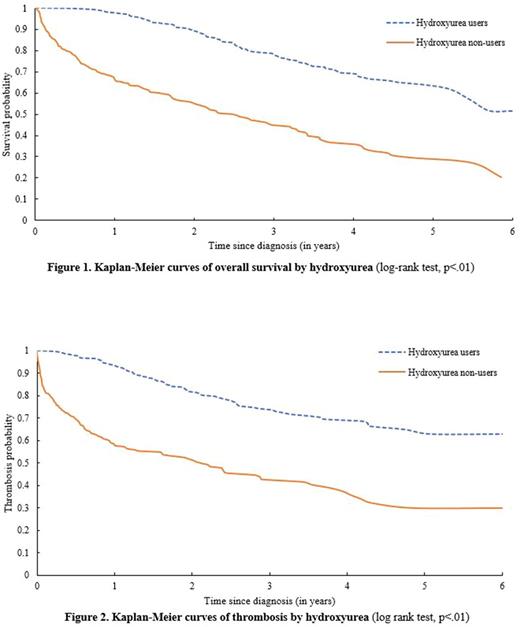
Results: A total of 654 high-risk ET patients with a mean age of 79.0 (standard deviation =7.4) years were included in the study, of which 67.9% were women and 85.5% were white. The median duration of follow-up was 2.5 years. In this large sample of older patients, only 59.2% of the patients received HU after ET diagnosis. Results of multivariate logistic regression models showed that patients with comorbidities (p<.01), with pre-existing cardiovascular conditions (p=.05), or disabled (p<.01) were less likely to receive HU. At the end of follow-up, 26.6% (n=103) HU users and 58.8% (n=157) non-users died. The median survival for HU non-users was 2.53 years, while it was not reached for HU users (log-rank test, p<.01, Figure 1). In the multivariate Cox model, HU usage after diagnosis was associated with a significantly lower risk of death (hazard ratio [HR]=0.42, 95% confidence interval [CI]: 0.32-0.54, p<.01). Every 10% increase of HU coverage was associated with a 16% decreased risk of death (HR=0.84, 95% CI: 0.81-0.88, p<.01). A total of 235 (35.9%) patients had thrombotic events after diagnosis, including 180 arterial thromboses, 54 venous thromboses and 1 sudden death. Thrombotic events occurred in 25.3% (n=85) HU users and nearly half of the HU non-users (47.2%, n=150). The median time to develop thrombosis was not reached for HU users and was significantly longer than for HU non-users (2.12 years, log-rank test, p<.01, Figure 2). In the multivariate model, compared with non-users, HU users had a significantly lower risk of thrombotic events (HR=0.51, 95% CI: 0.38-0.68, p<.01). Every 10% increase of HU coverage was associated with a 13% lower risk of thrombotic events (HR=0.87, 95% CI: 0.84-0.91, p<.01). Sensitivity analyses, which only included patients who survived more than 6 months, showed similar association between use of HU, improved survival and decreased incidence of thrombotic events.
Conclusions: To our knowledge, this is the first study to evaluate association between HU and survival among high risk ET patients. Use of HU was associated not only with improved overall survival but also with reduced incidence of thrombotic events in older ET patients. HU was underutilized in the studied high risk ET population, including patients with pre-existing cardiovascular conditions who need the drug the most.
Podoltsev: Incyte: Consultancy; CTI biopharma/Baxalta: Consultancy; Alexion: Consultancy; Ariad: Consultancy. Zeidan: Otsuka: Consultancy; AbbVie, Otsuka, Pfizer, Gilead, Celgene, Ariad, Incyte: Consultancy, Honoraria; Takeda: Speakers Bureau. Huntington: Celgene: Consultancy, Other: Travel; Janssen: Consultancy; Pharmacyclics: Honoraria. Ma: Incyte Corp.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal