Abstract
Introduction: Oncolytic virus therapy leads to immunogenic death of virus infected tumor cells. Evidence from mouse studies shows that this mode of tumor cell killing can boost the cytotoxic T lymphocyte response against tumor associated antigens (TAA), leading to 'bystander' killing of uninfected tumor cells.
Methods: To investigate whether oncolytic virotherapy can boost immune responses to tumor antigens in human subjects we studied T cell responses to 10 different TAA reported to have high expression in patients with multiple myeloma (n=10) before and after intravenous administration of an oncolytic measles virus (MV-NIS). Baseline and 6 week post-therapy PBMC samples were frozen at the time of collection from each enrolled patient throughout the course of the clinical trial. After thawing, 2 x 105 PBMC were stimulated with peptide pools specific to each of the selected TAA and their responses to this antigenic stimulation were measured using IFNγ ELISPOT. Spots more than 50 were counted as positive response.
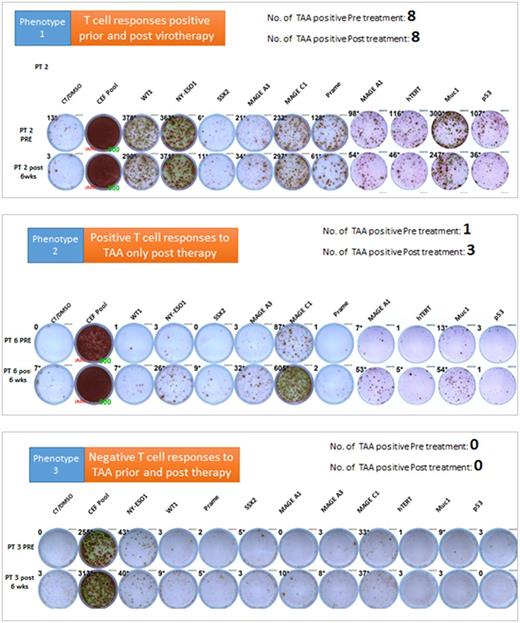
Results: Despite their prior exposure to multiple immunosuppressive anti-myeloma treatment regimens, some of the tested patients had higher T cell responses to some of the TAA even prior to Measles virotherapy. Measurable baseline T cell responses against MAGEc1 were present in 50% of the patients, and against hTERT and NYESO-1 in 30% of the patients for each target. Furthermore, MV-NIS treatment significantly boosted T cell responses against NYESO-1, MAGEc1, MAGEA1 and MUC1 in 40% of the patients. Interestingly, in one outlier patient who achieved a stringent and durable complete disease remission after MV-NIS therapy, strong baseline T cell responses were present against 8 of the 10 tested TAA but did not change significantly after virotherapy. Based on the T cell responses to the TAA, three distinct phenotypes can be identified. Phenotype 1 was characterized by positive T cell responses to TAA prior and post therapy, Phenotype 2 was characterized by positive T cell responses to TAA only post therapy and phenotype 3 was characterized by negative T cell responses to TAA prior and post therapy (Figure 1). Additionally, these observed phenotypes in T cell responses correlated with anti-tumor response.
Conclusion: Our data suggests oncolytic measles virotherapy boosted the cytotoxic T lymphocyte response against tumor associated antigens in some of the patients. Identifying patient's phenotype based on responsiveness to the TAA could be useful marker for identifying the immune responsive state in multiple myeloma patients and identify patients who can be most responsive to oncolytic measles virotherapy. Additionally, the boosted T cell responses against TAA post measles virotherapy provide a good rationale for combining oncolytic virotherapy with checkpoint antibody therapy to increase the efficacy of the oncolytic measles virotherapy.
Figure 1: T cell response phenotypes against 10 different TAAs. Representative IFNγ ELISPOT data shown below. PRE: T cell responses prior to virotherapy; Post 6 wks: T cell responses 6 weeks post virotherapy
Dispenzieri: Celgene, Millenium, Pfizer, Janssen: Research Funding. Peng: Imanis Life Sciences: Employment, Equity Ownership; Vyriad: Equity Ownership. Russell: Vyriad: Equity Ownership; Imanis Life Sciences: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal