Abstract
Introduction: First generation PI3Kδ inhibitors such as idelalisib and duvelisib are active in patients (pts) with lymphoid malignancies but are often associated with significant immune-mediated adverse events, including transaminitis, diarrhea/colitis, and pneumonitis, as well as an increased risk of serious infections. These toxicities can be severe, and frequently lead to treatment discontinuation. Umbralisib (TGR-1202) is a next generation, once-daily, oral PI3Kδ inhibitor, that is active in pts with relapsed/refractory (R/R) lymphoid malignancies, with a 94% ORR in previously treated CLL (Burris et al, 2015). Umbralisib has a markedly different chemical structure and pharmacologic profile compared to idelalisib and duvelisib, and kinome-profiling further differentiates umbralisib from these agents. All three compounds are selective for the PI3Kδ isoform, however the PI3Kγ isoform, which promotes pro-inflammatory cytokines and increased recruitment and cytotoxicity of T cells, is not inhibited by umbralisib (Kaneda et al, Nature 2016). Umbralisib also has an additional effect on casein kinase-1 epsilon (CK-1ε), a protein which may have an inhibitory effect on regulatory T-cell function (Deng et al, 2016). Collectively, these pharmacologic features provide a potential rationale for the differentiated toxicity profile of umbralisib compared to other PI3Kδ inhibitors. Here, we present an integrated safety analysis for pts dosed with umbralisib either as monotherapy or in combination with other agents.
Methods: Safety data were pooled from 5 completed or ongoing Phase 1 or 2 studies containing umbralisib. All studies shared similar key eligibility criteria: pts had R/R lymphoid malignancies with an ECOG PS ≤ 2 without limit to number of prior therapies. Umbralisib was dosed daily until progression or unacceptable toxicity, while dosing of combination agents varied. Adverse event grading was done by CTCAE v4.03 criteria.
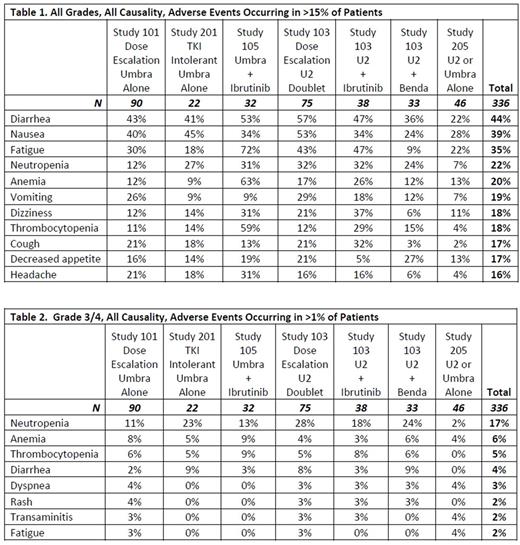
Results: A total of 336 patients were included in the analysis. Patients received the following regimens: umbralisib as monotherapy (135 pts) or umbralisib in combination with: the glycoengineered anti-CD20 mAb ublituximab ("U2", 98 pts), ibrutinib (32 pts), ublituximab + ibrutinib (38 pts), or ublituximab + bendamustine (33 pts). Among the 336 patients, 32% had CLL/SLL, 35% DLBCL, 22% indolent NHL, and 12% other lymphoma. Median and mean duration of exposure to umbralisib was 5 and 9 months respectively, with the longest patient on daily umbralisib for 4+ years, and a cumulative duration of drug exposure across all 336 patients of over 240 years. Common all-grade adverse events regardless of causality are described in Table 1, and Grade 3/4 adverse events are described in Table 2. The most common all-grade non-hematologic toxicities were: diarrhea (44%), nausea (39%), and fatigue (35%). All-grade hematologic toxicities included neutropenia (22%), anemia (20%), and thrombocytopenia (18%). Grade 3/4 adverse events were infrequent, with the most common non-hematologic events including diarrhea (4%) and dyspnea (3%), and hematologic toxicities of neutropenia (17%), anemia (6%), and thrombocytopenia (5%). Key adverse events prevalent amongst prior generation PI3Kδ inhibitors were also infrequent: transaminitis (9% All; G3/4 <3%), colitis (<1.5% All; G3/4 <1%), and pneumonitis (<1.5% All; G3/4 <0.5%). Serious adverse events that occurred in >1% of patients were pneumonia (5%), febrile neutropenia (3%), sepsis (2%), and pyrexia (2%). Discontinuations due to adverse events were rare at under 10% for all studies.
Conclusions: In this integrated safety analysis of 336 patients, many of whom were on treatment for over a year, umbralisib exhibits a differentiated safety profile compared to prior generation PI3kδ inhibitors. Improved tolerability with very few discontinuations of umbralisib due to AEs has allowed patients to remain on continuous dosing to achieve and sustain promisingly high rates of response. The mechanism for decreased immune-mediated toxicity is still being elucidated through ongoing pre-clinical and correlative studies examining umbralisib's selectivity for PI3Kδ over PI3Kγ, complimentary CK1ε inhibition, and enhancement of regulatory T-cell function.
Davids: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy; Merck: Consultancy; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Infinity: Consultancy, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; InCyte: Membership on an entity's Board of Directors or advisory committees. Flinn: Beigene: Research Funding; Agios: Research Funding; Janssen: Research Funding; Constellation: Research Funding; Takeda: Research Funding; Forty Seven: Research Funding; Infinity: Research Funding; Portola: Research Funding; Janssen: Research Funding; Verastem: Research Funding; Genentech: Research Funding; Novartis: Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; KITE: Research Funding; Pfizer: Research Funding; TG Therapeutics: Research Funding; Pharmacyclics LLC: Research Funding; Seattle Genetics: Research Funding; Celgene: Research Funding; Curis: Research Funding; Trillium: Research Funding; AbbVie Company: Research Funding; Acerta: Research Funding; Calithera: Research Funding; Incyte: Research Funding; Gilead: Research Funding. Mato: Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; DTRM: Research Funding; Portola: Research Funding; Acerta: Research Funding; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy; AstraZeneca: Consultancy; Kite: Consultancy; Pharmacyclics: Research Funding; Regeneron: Research Funding. O'Connor: Celgene: Honoraria, Research Funding; Trillium Therapeutics: Research Funding. Brander: Genentech: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Teva Pharmaceuticals, Genentech, AbbVie, Pharmacyclics: Consultancy; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lunning: Juno: Consultancy; AbbVie: Consultancy; Pharmacyclics: Consultancy; Celgene: Consultancy; Onyx: Consultancy; Gilead: Consultancy; TG Therapeutics: Consultancy; Genentech: Consultancy; Epizyme: Consultancy; Spectrum: Consultancy; BMS: Consultancy. Nastoupil: Celgene: Honoraria, Research Funding; Karus Therapeutics: Research Funding; Genentech: Honoraria, Research Funding; Gilead: Honoraria; Abbvie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; TG Therapeutics: Honoraria, Research Funding. Flowers: Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; Millennium/Takeda: Research Funding; OptumRx: Consultancy; Pharmacyclics LLC, an AbbVie Company: Research Funding; Bayer: Consultancy; Prime Oncology: Research Funding; Gilead: Consultancy; Research to Practice: Research Funding; Educational Concepts: Research Funding; Abbvie: Consultancy, Research Funding; Spectrum: Consultancy; National Institutes Of Health: Research Funding; Janssen Pharmaceutical: Research Funding; Celgene: Consultancy, Research Funding; TG Therapeutics: Research Funding; Acerta: Research Funding; Clinical Care Options: Research Funding; V Foundation: Research Funding; Burroughs Welcome Fund: Research Funding; Seattle Genetics: Consultancy; Onyx: Research Funding; Genentech/Roche: Consultancy, Research Funding; Infinity: Research Funding. Brown: Celgene: Consultancy; AbbVie: Consultancy, Honoraria; AstraZeneca: Consultancy; Janssen: Consultancy; Janssen Oncology: Honoraria; Infinity Pharmaceuticals: Consultancy; Sun BioPharma: Consultancy, Research Funding; Pharmacyclics: Consultancy; Astellas Pharma: Consultancy; Roche/Genentech: Consultancy; Pfizer: Consultancy; Redx: Consultancy; Gilead: Consultancy, Research Funding. Ghosh: Pharmacyclics: Consultancy, Honoraria, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Jassen: Consultancy, Honoraria, Research Funding; Kite Pharma: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria, Research Funding. Lansigan: Spectrum Pharmaceuticals: Consultancy, Research Funding; Seattle Genetics: Consultancy. Cheson: AbbVie, Roche-Genentech, Pharmacyclics, Acerta: Consultancy; Acerta, Pharmacyclics, Epizyme, Gilead, Roche, AbbVi: Other: Institution receives research support . Barr: AbbVie: Consultancy, Research Funding; Novartis: Consultancy; Celgene: Consultancy; Gilead: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Seattle Genetics: Consultancy; Infinity: Consultancy. Pagel: Actinium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Pinilla-Ibarz: ARIAD: Consultancy, Honoraria; BMS: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau. Burke: Celgene: Consultancy; Bayer: Consultancy; Incyte: Consultancy; Gilead: Consultancy; Genentech: Consultancy. Siddiqi: Pharmacyclics, an AbbVie Company: Other: Steering committee for ibrutinib, Speakers Bureau; Juno: Other: Steering committee for JCAR017; Seattle Genetics: Speakers Bureau. Patel: BMS: Speakers Bureau; Gilead: Speakers Bureau; Genentech: Speakers Bureau; Exelixis: Speakers Bureau; Medivation: Speakers Bureau. Gribben: Pharmacyclics: Honoraria; Janssen: Honoraria; Genentech/Roche: Honoraria; Kite: Honoraria; Acerta: Honoraria; TG Therapeutics: Honoraria; Karyopharm: Honoraria; Abbvie: Honoraria; Celgene: Honoraria. Zinzani: Merck: Consultancy, Other: Advisory board; Celgene, Roche, Janssen, Gilead, Takeda, BMS, MSD, Servier, Sandoz, Mundipharma: Honoraria; Celgene, Janssen, Gilead, Roche, Takeda, BMS, MSD, Sandoz, Servier, Mundipharma: Speakers Bureau. Miskin: TG Therapeutics, Inc.: Employment, Equity Ownership. Sportelli: TG Therapeutics, Inc.: Employment, Equity Ownership. Weiss: TG Therapeutics, Inc.: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. O'Brien: AbbVie: Consultancy; Regeneron: Other: Research Support: Honorarium, Research Funding; ProNAI: Other: Research Support: Honorarium, Research Funding; Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; Sunesis: Consultancy; Pfizer: Consultancy, Research Funding; Astellas: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Acerta: Other: Research Support: Honorarium, Research Funding; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Aptose Biosciences, Inc.: Consultancy; Alexion: Consultancy; Vaniam Group LLC: Consultancy; GSK: Consultancy; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal