Abstract
The Berlin-Frankfurt-Münster (BFM) based protocols have been wildly used in children with non-Hodgkin lymphoma (NHL) and leukemia for more than ten years in China. At the same time, rituximab has become a standard medicine in the treatment of adult patients with diffuse large B-cell lymphoma (DLBCL) and relapsed or refractory B-cell NHL. In this study, we explored the efficacy of rituximab in childhood CD20 positive B-NHL and B-cell acute leukemia (B-AL).
To explore the effect of rituximab in mature B-cell lymphoma (B-NHL)/mature B-cell leukemia ((B-AL) in children, this study included children and adolescents aged younger than 18 years with newly diagnosed CD20 positive, stage III/IV B-NHL/B-AL for treatment combining NHL-BFM95-based protocol with rituximab. A total of 4 injections with 375 mg/m2 rituximab each were administered intravenously after prephase V course at one day before the following 4 course of chemotherapy. Retrospective cases diagnosed with stage III/IV B-NHL/B-AL in our center were used as historical control. All of these patients were treated under NHL-BFM90 based protocol. Patient characteristics, event free survival (EFS), recurrence and mortality were analyzed.
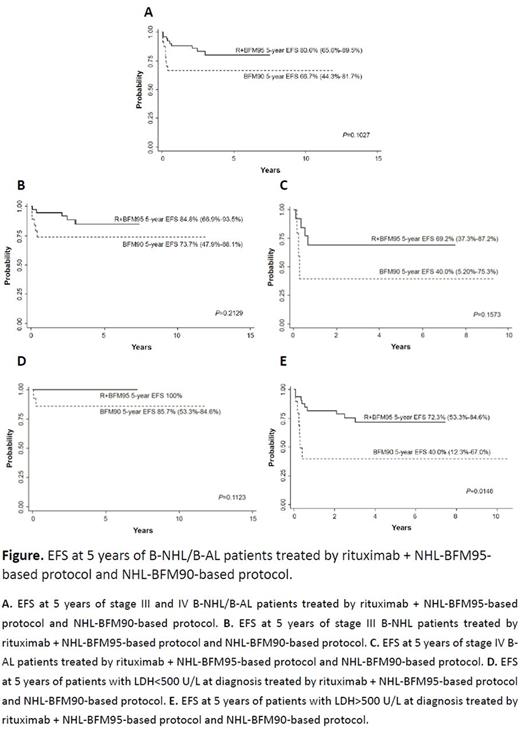
From April 2009 to December 2015, 46 patients were recruited and treated with rituximab combining BFM95-based protocol (R+BFM95 protocol). 23 cases diagnosed with stage III/IV B-NHL/B-AL from January 2005 to March 2009 treated with BFM90-based protocol. All patients followed till December 31, 2016. This study recruited 35 males and 11 females with an average age of 6.9 years who were treated with R+BFM95 protocol. Among the historical control of 23 patients treated with BFM90-based chemotherapy, 18 were males and 5 were females and the average age of our historical control was 7.2 years. Among patients treated with R+BMF95 protocol, the average duration of continuous complete remission (CR) was 3.6 years. Compared with patients treated with BFM90-based protocol, the 5-year event free survival (EFS) of patients under R+BMF95 was higher (83.7±5.7% vs 69.6%±9.6% in R+BMF95 and BFM90 respectively), however the difference was not statistically significant (P=0.1062). Among subgroups of our patients between patients treated with R+BMF95 and BFM90, the 5-year EFS of patients with stage III disease was 87.3±6.1% vs 77.8±9.8% (P=0.2998), stage IV subgroup was 72.7%±13.4% vs 40.0±21.9% (P=0.0878), respectively. Among patients whose LDH level were below 500 U/L at diagnosis, R+BFM95 protocol achieved 100% EFS during our follow-up, nevertheless the 5-year EFS of patients in this group was not statistically different from that of patients treated with BFM90 (92.3±7.4%, P=0.2994). Among patients had LDH >500 U/L at diagnosis, the 5-year EFS in R+BFM95 group was 77.2±7.7% and significantly higher than that of BFM90 group (40.0±15.5%, P=0.0048). In the patient group treated with R+BFM95, there were 2 (2/46, 4.3%) did not achieve CR. During the follow-up, recurrence occurred in 5 patients (5/46, 10.9%) including 2 partial remission (PR). Early death occurred in 1 patient (1/46, 2.2%) in the first course due to tumor lysis syndrome. Two cases with recurrence were treated with R-ICE regimen however remission did not occur. All patients with recurrent disease died. In the control patients treated with BFM90-based protocol, there were 3 (3/23, 13.0%) did not achieve CR. One patient did not respond to chemotherapy therefore received second-look operation however the pathology was positive and he died of disease progression. The other 2 patients give up treatment after 2 courses of chemotherapy. One patient (1/23, 4.3%) died in the first course because of sepsis. One patient with relapsed disease received R-ICE treatment however not achieved remission again. All patients with recurrent disease also died in BFM90 group.
To summarize, our study demonstrated the feasibility of rituximab for Chinese children with B-NHL/B-AL. Rituximab improved the prognosis of Chinese B-NHL/B-AL children, especially among patients with LDH >500U/L. However, the limited number of patients and retrospective study design suggested further randomized trial with larger number of patients to confirm the efficacy, as well as cost-effectiveness of rituximab in childhood B-NHL/B-AL in China.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal