Abstract
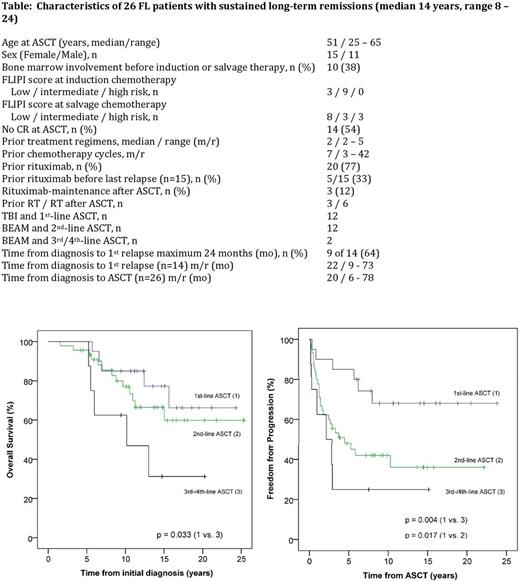
Background: Long-term clinical and molecular remissions in patients with FL following HDT and ASCT have been evaluated in only a few studies. Results are especially limited for second-line HDT with BEAM (BCNU, etoposide, cytarabine and melphalan). Patients and methods: 73 patients with FL were treated in our institution from 1993 to 2016 (20 1st-line ASCT with total body irradiation (TBI) and cyclophosphamide, 46 2nd-line with BEAM and 8 ≥3rd-line with BEAM). Patients with high-grade transformation before ASCT were excluded. The data were collected retrospectively in 44 and prospectively in 29 patients who participated in two clinical trials: the 1st-line therapy trial of the German Low Grade Lymphoma Study Group (GLSG, principal investigator W. Hiddemann [Lenz G, Blood 2004]) and the salvage therapy LYM1 trial of the EBMT [Pettengell R, JCO 2013]. For the salvage ASCT protocol, most of the patients received 3 - 4 courses of the ESHAP or ICE protocol-since 2001, also usually combined with rituximab. Stem cell apheresis was carried out in the Blood Transfusion Service following these protocols or cyclophosphamide, usually in remission. Patients with partial remission after ASCT received a radiotherapy (RT) with 30-36 Gy in the field of persisting lymphoma, if possible, except participants of the LYM1 trial. Further details are described in an earlier publication of our first 60 patients (Metzner B, Ann Oncol 2013). Response assessment was performed by careful clinical examination (with ultrasound, chest x-ray and partly CT) at regular 3 - 12 monthly time points. In the case of long-term remission (≥ 8 years; n = 26), peripheral blood was tested for minimal residual disease by quantitative t(14;18) RQ-PCR and/or IGH-consensus PCR (n = 25). Results: With a median follow-up of 9.4 years (range 1.2-23.8) 10-year overall survival, progression-free survival and freedom from progression (FFP) after 1st-line ASCT were 80%, 60% and 68%, after 2nd-line ASCT 58%, 37% and 42%, after ≥3rd-line ASCT 50%, 25% and 25%, respectively, with a significant difference for OS, PFS and FFP between 1st-line and ≥3rd-line cohort, for PFS and FFP between 1st- and 2nd-line cohort (p<0.05). The median OS from the initial diagnosis for the 1st-line cohort and the 2nd-line cohort was not yet reached (estimated >23 years); and 9.4 years for the ≥3rd-line cohort; for a subgroup of the 2nd-line cohort with early relapse (time from diagnosis to 1st relapse maximum 24 months, n = 26) not yet reached (estimated >21 years). Ten-year FFP for 2nd-line ASCT and low-risk FLIPI score (n = 16) was 69%, intermediate risk (n = 11) 27% and high risk (n = 18) 29% (p=0.051). No relapses occurred after 8 years following ASCT in 1st or 2nd remission (except one patient 10 years after ASCT). So far, 26 patients developed long-term remissions of at least 8 years (up to 24, median 14 years). 24 of the 25 tested long-term remission patients were MRD-negative at the last follow-up. One patient was MRD-positive (low-level MRD) at the last control (10.6 years after ASCT in 1st line, unchanged as the years before). Five-year non-relapse mortality was 0%. Four of 73 patients developed secondary invasive malignancies in remission after ASCT: in the TBI cohort, 2 of 20 patients; in the BEAM cohort, 2 of 53 patients, (in the 2nd-line ASCT cohort 1 of 45 patients). Conclusion: Sustained long-term clinical and molecular remissions up to > 22 years can be achieved following ASCT in 1st or 2nd treatment line, indicating the potential curative impact of ASCT in FL.
Pott: Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal