Abstract
Background
Micafungin is a tolerable and effective prophylactic antifungal agent used during allogeneic stem cell transplantation. In this prospective single arm trial, we aimed to evaluate the potential efficacy and safety of micafungin as a prophylactic agent during first induction chemotherapy in acute leukemia patients.
Materials and Methods
Patients received 50mg of micafungin intravenously once daily from initiation of induction chemotherapy to recovery of neutrophil count (absolute neutrophil count > 500/μg for consecutive days) or suspected fungal infection or occurrence of drug-related toxicity. Primary end point was the incidence of invasive fungal infection and secondary end point was overall survival and non-relapse mortality (ClinicalTrials. Gov number, NCT02440178).
Result
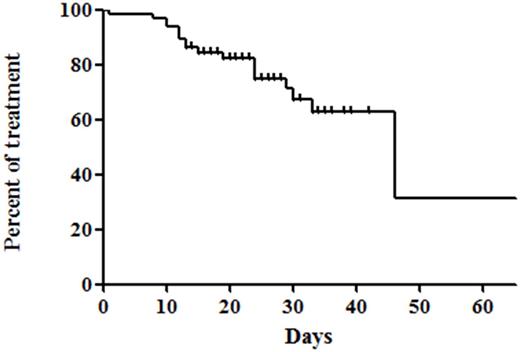
A total of 65 patients (median age 51.0 years, male:female = 34:31) were enrolled in this study; 33 (50.8%) acute myeloid leukemia (AML), 31 (47.7%) acute lymphoblastic leukemia, and 1 acute biphenotypic leukemia, respectively. The median treatment duration of micafungin was 24 days (range 1.0-68 days). Proven invasive fungal disease occurred in 1/65 patients (1.5%) and possible fungal infection occurred in 2/65 patients (4.6%). Overall, 19/65 patients (29.2%) discontinued prophylactic micafungin and 18/65 patients (27.7%) changed micafungin to other antifungal agents; 2 patients (3.1%) for fungal infection and 15 patients (23.1%) for prolonged neutropenic fever of unknown cause. For safety, 3/65 patients (4.6%) experienced adverse events: 2 liver function abnormality (Grade 2 and 3 respectively) and 1 grade 2 allergic reaction. Induction-related mortality occurred in 3 patients, where 1 event was caused by invasive aspergillosis pneumonia. The period of from initiation of micafungin to change to other antifungal agents was 46 days (95% CI 27-64 days). There were no significant differences in adverse events and fungal infections between AML and ALL (2/33 for AML vs. 1/31 for ALL patients, p=1.000). In addition, the occurrence of fungal infection during induction therapy was associated with poor overall survival (hazard ratio 8.470, 95% confidence interval 1.048-68.465, p=0.045) in multivariable analysis. Additionally, there was no fungal infection caused by resistant organism after induction chemotherapy. Micafungin also prevented invasive fungal infection more effectively and adverse events were tolerable when compared to the results of previous prophylactic posaconazole trial (proven or probable invasive fungal infection: 2% (7/304); adverse events 19/304 (6%) in patients treated with prophylactic posaconazole) [1].
Conclusion
Likewise posaconazole, micafungin could be used effectively and safely as prophylactic regimen in both AML and ALL patients undergoing induction chemotherapy.
Reference
1. Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. The New England journal of medicine. 2007;356:348-59.
Figure caption
Figure 1. The period of from initiation of micafungin to change to other antifungal agents
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal