Abstract
Background: Sickle cell trait (SCT) is common, affecting 7-8% of African Americans. Previously thought to be of little clinical consequence except under conditions of severe hypoxic/metabolic stress, recent studies have shown that SCT is independently associated with chronic vascular diseases including kidney disease, coronary artery disease, overt stroke, and pulmonary embolism. It has been postulated that SCT underlies the increased prevalence of cerebrovascular disease in African-Americans after adjusting for excess risk factors. Prospective imaging studies evaluating the natural history of cerebral ischemia in SCT are lacking; thus, the true disease burden is unknown. In this study, we hypothesized that individuals with SCT are at increased risk of silent cerebral infarctions (SCI) compared to healthy young adults, but with a lower risk of SCIs compared to young adults with sickle cell anemia (SCA). We further evaluated and compared the patterns of SCIs within SCT and SCA.
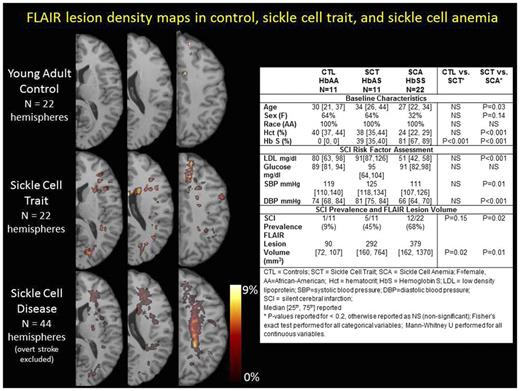
Methods: Three cohorts were prospectively recruited from a tertiary care referral center for pediatric and adult SCA: (1) young adults with SCA, (2) healthy, young adults with SCT, and (3) healthy, age- and race-matched, controls. Control and SCT subjects were excluded for history of hypertension, diabetes, anemia, dyslipidemia, chronic medical or neurological illness, or severe trauma; individuals with SCA were excluded if on chronic transfusion therapy or history of overt stroke, vasculopathy, or chronic neurological illness. Brain MRI T1 and FLAIR maps were evaluated for the presence of two lesion types (defined in Wardlaw et al, Lancet Neurol, 2013): (a) FLAIR lesions were defined as FLAIR hyperintensity > 3mm in diameter; (b) SCIs were defined as FLAIR lesions with the additional requirement of CSF-like hypointensity on T1. Thus, SCIs represented a subset of all FLAIR lesions. FLAIR lesions were outlined by a vascular neurologist using MIPAV (Medical Image Processing, Analysis and Visualization, https://mipav.cit.nih.gov/) from which individual lesion volumes were calculated. Baseline characteristics, prevalence of SCIs, and volume of FLAIR lesions in SCT were compared to controls and SCA (Fishers Exact for categorical variables; Mann-Whitney U for continuous variables). To evaluate the distribution of SCIs in SCT relative to healthy controls and SCA, three lesion density maps were created. Lesions drawn on FLAIR 5mm slice thickness were stretched in the z-direction to 10mm for display of SCI pattern on few slices.
Results: Control, SCT, and SCA adults were similar with respect to baseline characteristics, except SCA subjects were younger and fewer female than SCT (Table). Ten (45%) of the SCA subjects were on hydroxyurea. We acquired LDL, glucose, and blood pressure to ensure that SCT participants did not have higher risk of SCIs compared to controls due to ongoing comorbidities (Table). Imaging Outcomes: We found greater FLAIR lesion volumes in SCT than controls (292 vs. 90 mm3, p=0.02) and a trend towards greater prevalence of SCIs -- 5 of 11 (45%) in SCT compared to 1 of 11 (9%) in controls (p=0.15). FLAIR lesion volumes and SCI prevalence were higher in SCA compared to SCT (p=0.01 and p=0.02, respectively). Lesion density maps (Figure) were created to evaluate SCI patterns in SCT and SCA. In the SCA cohort, lesion density was highest within the internal borderzone (where cerebral blood flow is at a nadir), while this region was relatively unaffected in SCT. In contrast, a distinct pattern of small cortical/subcortical strokes distributed in a stochastic pattern (not following any particular vascular territory) were found in both SCT and SCA.
Conclusions: We found greater FLAIR lesion volumes and a trend towards a greater prevalence of SCIs in healthy individuals with SCT. We postulate that SCIs found in SCT may represent an etiologic subtype of SCIs found in SCA, representing microembolic events or a microvasculopathy, in contrast to the etiology of internal borderzone infarction which likely represents a heightened ischemic vulnerability within low CBF regions in the setting of chronic, severe anemia. Larger studies are necessary to determine if SCT carries a heightened risk of silent cerebral ischemia, which would impact society at large given the high prevalence of SCT and the known effects of SCIs on cognitive disability.
Fields: Proclara Biosciences: Equity Ownership. Hulbert: Pfizer: Other: Spouse employment at Pfizer.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal