Abstract
Introduction: Although increased physical activity (PA) is associated with improved quality of life in cancer survivors, knowledge about the impact of PA on survival in lymphoma patients is limited. We studied the impact of usual adult PA and PA at 3 years after diagnosis on overall (OS) and lymphoma-specific (LSS) survival in lymphoma patients.
Methods: Lymphoma patients were prospectively enrolled within 9 months of diagnosis into the Lymphoma SPORE Molecular Epidemiology Resource (MER) cohort. At baseline, patients reported their usual level of mild, moderate and strenuous PA during most of their adult life and (for survivors) again at the 3 year follow-up (FU3), which we used to calculate the Godin Leisure Score Index (LSI). At FU3, patients also self-reported change in PA since diagnosis as increase, decrease or no change. LSI was modeled as a continuous score (per 10-point change) and by tertile. Survival was measured as time from diagnosis (for baseline PA) and time from FU3 (for FU3 PA) until death due to any cause and due to lymphoma. We evaluated the association of PA with outcome using Kaplan-Meier curves as well as hazard ratios (HRs) and 95% confidence intervals (CI) from Cox models stratified by lymphoma subtype and adjusted for age and sex (and baseline PA for change models).
Results: Of 4087 patients enrolled in the MER at the Mayo Clinic from 2002-2012, 3129 had baseline PA data (3060 evaluable for LSI), and 1845 (1395 evaluable for LSI) had FU3 PA data. In the baseline cohort, 57.6% of the patients were male. The median age at diagnosis was 62 years (range 18-92 years). The majority had ECOG performance status <2 (95%), no B-symptoms (85.8%) and advanced disease (63% stage III-IV) at diagnosis. The subtypes included were CLL/small lymphocytic (25.9%), follicular (18.1%), diffuse large B-cell (16.8%), marginal zone (8.1%), Hodgkin (6.9%), mantle cell (4.8%), T-cell (5%) and other (14.3%) lymphomas. The median LSI was 28 (range 0-57) at baseline and 25 (range 0-67) at FU3 with a median change in LSI of -2 (range -57 to 61). At a median follow-up of 84 months from diagnosis (IQR 59-119 months) in the cohort with baseline PA, there were 806 total deaths (271 in pts with FU3 PA) and 423 lymphoma deaths (104 in pts with FU3 PA).
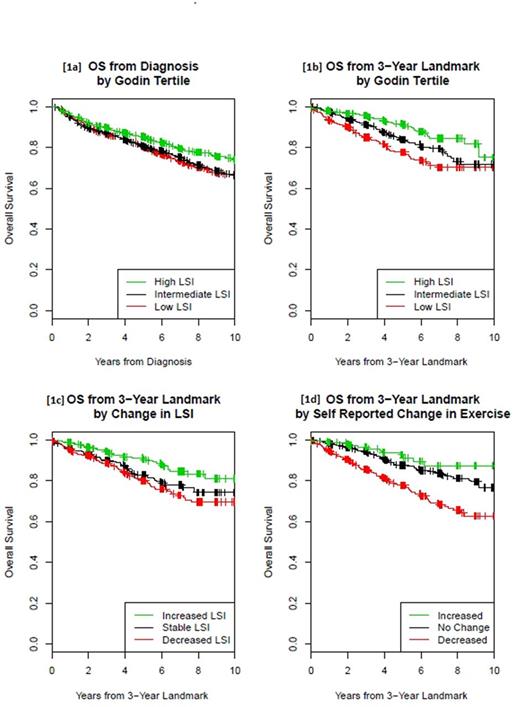
When analyzed as a continuous variable, baseline LSI was associated with OS (HR 0.95, 95% CI 0.91-0.99) and LSS (HR 0.95, 95% CI 0.90-1.01). LSI by tertiles was associated with OS (p<0.0001, Figure 1a), and patients in the high LSI compared to the low LSI tertile had superior OS (HR 0.81, 95% CI 0.68-0.97) and LSS (HR 0.81, 95% CI 0.63-1.04). For prognosis after 3 years, continuous LSI at FU3 was also associated with superior OS (HR 0.84, 95% CI 0.77-0.92) and LSS (HR 0.76, 95% CI 0.65-0.89). LSI at FU3 by tertiles was associated with OS (p<0.0001, Figure 1b), and patients in the high LSI compared to the low LSI tertile had superior OS (HR 0.51, 95% CI 0.34-0.77) and LSS (HR 0.33, 95% CI 0.16-0.67).
A change in LSI from baseline to FU3 PA was associated with superior OS (HR 0.84, 95% CI 0.76-0.92) and LSS (HR 0.74, 95% CI 0.64-0.87) after FU3. Change in LSI by tertiles was associated with OS (p=0.0007, Figure 1c). Compared to patients with stable LSI (change of LSI between -10 and 5 points from baseline, n=429), patients who increased LSI by > 5 (n=464) had improved OS (HR 0.67, 95% CI 0.45-1.00) and LSS (HR 0.47, 95% CI 0.23, 0.94), while patients who decreased LSI by >10 (n=477) had no association with OS or LSS. Self-reported change in PA from baseline to FU3 was associated with OS (p<0.0001, Figure 1d); compared to no change, an increase in PA was not associated with OS (HR 0.79, 95% CI 0.42-1.48) or LSS (OR 0.73, 0.26-2.07), while a reduction in PA was associated with inferior OS (HR 1.94, 95% CI 1.49-2.53) and LSS (HR 2.51, 95% CI 1.62-3.88). After excluding patients with any events through FU3, the associations for the PA change and OS remained significant for change in LSI (HR 0.91, 95%CI 0.83-1.00) and self-reported decrease in PA (HR 1.83, 95%CI 1.32-2.53).
Conclusions: Patients with a higher level of usual PA during adult life have significantly better OS and LSS after lymphoma diagnosis compared to those who are less physically active. Higher PA in 3-year survivors is also associated with improved survival, while a reduction in physical activity is associated with worse outcomes. These data support an important role for PA in lymphoma survivorship and also provide a strong rationale for a PA intervention trial in lymphoma patients.
Cohen: LAM Therapeutics, Inc: Research Funding; Takada: Research Funding; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bioinvent: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees. Flowers: Spectrum: Consultancy; OptumRx: Consultancy; Acerta: Research Funding; Onyx: Research Funding; Janssen Pharmaceutical: Research Funding; V Foundation: Research Funding; TG Therapeutics: Research Funding; National Cancer Institute: Research Funding; Burroughs Welcome Fund: Research Funding; Genentech/Roche: Consultancy, Research Funding; Gilead: Consultancy; Research to Practice: Research Funding; Millennium/Takeda: Research Funding; National Institutes Of Health: Research Funding; Prime Oncology: Research Funding; Educational Concepts: Research Funding; Abbvie: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Seattle Genetics: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Celgene: Consultancy, Research Funding; Infinity: Research Funding; Bayer: Consultancy; Clinical Care Options: Research Funding. Cerhan: Janssen: Other: Scientific Advisory Board (REMICADELYM4001); Janssen: Other: Multiple Myeloma Registry Steering .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal