Key Points
The spleen but not bone marrow microenvironment induces CD44v6 variants in CLL, which promote early engraftment.
CD44v6 expression is linked to NF-κB and MAPK signaling in murine and human B-cell leukemia and contributes to proliferation.
Abstract
Chronic lymphocytic leukemia (CLL) outgrowth depends on signals from the microenvironment. We have previously found that in vitro reconstitution of this microenvironment induces specific variant isoforms of the adhesion molecule CD44, which confer human CLL with high affinity to hyaluronan (HA). Here, we determined the in vivo contribution of standard CD44 and its variants to leukemic B-cell homing and proliferation in Tcl1 transgenic mice with a B-cell–specific CD44 deficiency. In these mice, leukemia onset was delayed and leukemic infiltration of spleen, liver, and lungs, but not of bone marrow, was decreased. Competitive transplantation revealed that CLL homing to spleen and bone marrow required functional CD44. Notably, enrichment of CD44v6 variants particularly in spleen enhanced CLL engraftment and proliferation, along with increased HA binding. We recapitulated CD44v6 induction in the human disease and revealed the involvement of MAPK and NF-κB signaling upon CD40 ligand and B-cell receptor stimulation by in vitro inhibition experiments and chromatin immunoprecipitation assays. The investigation of downstream signaling after CD44v6-HA engagement uncovered the activation of extracellular signal-regulated kinase and p65. Consequently, anti-CD44v6 treatment reduced leukemic cell proliferation in vitro in human and mouse, confirming the general nature of the findings. In summary, we propose a CD44-NF-κB-CD44v6 circuit in CLL, allowing tumor cells to gain HA binding capacity and supporting their proliferation.
Introduction
The pathophysiology of chronic lymphocytic leukemia (CLL) heavily depends on the tumor microenvironment.1 CLL cells that circulate in peripheral blood (PB) lack intrinsic proliferative capacity. However, this quiescence can switch to proliferation once leukemia cells infiltrate lymphoid organs and receive activating signals by the microenvironment.2 Novel kinase inhibitors such as ibrutinib disturb this communication, with great clinical success.3 They reduce CLL cell proliferation within lymphoid organs and mobilize leukemic cells into the periphery, preventing their further activation. However, how proliferative and adhesive signals cooperate in CLL is not understood yet.
CD44 comprises a set of transmembrane glycoproteins that are required for many cellular functions, including adhesion and activation. CD44 was 1 of the first described homing receptors and has been suggested as a cancer stem cell marker in various tumors.4,5 The Cd44 gene encodes a multitude of CD44 isoforms (variants) collectively termed CD44v, which are generated by alternative splicing of up to 10 variant exons between exon 5 and 6 of the CD44 standard isoform (CD44s).6 Resting lymphocytes express CD44s, whereas the alternative isoforms are induced by activation of the cells.6 In contrast, many tumor cells constitutively express CD44v.6 We previously observed that resting CLL cells display only minor levels of CD44v, but upon CD40L stimulation, glycosylated variants, particularly CD44v3 and CD44v6, are transcribed.7 This changes the binding affinity of CD44 to its major ligand hyaluronan (HA) and results in enhanced adhesive capacity of CLL cells to stromal cells.7
Signaling cascades involved in CD44v induction and functional consequences for CLL pathophysiology remained to be elucidated. Here, we addressed the in vivo contribution of CD44 and its high-affinity isoforms to CLL progression in an organ-specific manner by using a conditional B-cell–specific Cd44 knockout model on basis of the well-established Tcl1 transgenic (Tcl1-tg) CLL murine model. Tcl1-tg mice develop an aggressive CLL-like disease, in which CD5+/CD19+ expressing cells are first found in the peritoneal cavity (PC) at an age of 2 to 3 months, later followed by a spread of leukemic cells through the circulation and into the lymphoid organs.8 Removal of Cd44 on malignant cells in this model allowed us to define a key contribution of CD44v6 to leukemic B-cell proliferation, signaling, and CLL progression in vivo.
Methods
Mice
Tcl1-tg mice were obtained from Carlo Croce.8 CD19Cre mice9 (strain 006785) were purchased from Jackson Laboratories. Cd44flox/flox mice were described.10 Genotyping was performed by polymerase chain reaction (PCR), and CD44 deficiency was confirmed by flow cytometry. Leukemia onset and progression were monitored by regular flow cytometric tumor load quantification in PB. Absolute cell numbers were determined using Flow-Count Fluorospheres (Beckman Coulter) or the EVE automatic cell counter (NanoEnTek).
Patient samples
Antibodies and reagents
Antibodies are listed in supplemental Table 2.
Flow cytometry
Murine cells and human PBMCs were stained with specific antibodies (supplemental Table 2) or corresponding isotype controls. To detect intracellular antigens, cells were fixed and permeabilized with BD Cytofix/Cytoperm Kit (BD Biosciences). To detect HA binding, cells were incubated with HA-FITC (AbLab) or HA-TAMRA (Creative Peg Works) for 15 minutes at room temperature.
In all experiments, leukemic cells were identified by anti-CD5 and anti-CD19 stainings. Viable cells were identified using fixable viability dye. Measurements were performed using the FC-500 or Gallios system (Beckman Coulter).
Histology
Spleens, bone marrow (BM), lymph nodes, livers, lungs, and kidneys from Tcl1-tg mice and C57BL/6J wild-type mice with and without B-cell–specific CD44 deficiency were fixed in 4% formalin for 24 hours at room temperature, subsequently embedded in paraffin, cut into 4-µm sections, and stained with hematoxylin and eosin.
RT-PCR
Murine leukemic cells were selected with EasySep Mouse B-cell Isolation Kit (STEMCELL Technologies) for >96% purity. RNA isolation and complementary DNA (cDNA) synthesis were performed as described.11 For detection of CD44v transcription by reverse transcription PCR (RT-PCR), cDNA from leukemic cells was amplified by panCD44 or CD44 variant exon-specific primers and visualized by agarose gels. Primers are listed in supplemental Table 3.
Adoptive transfers
Splenocytes (0.5-15 × 106) were IV injected IV into C57BL/6J wild-type mice (Javier Laboratories). For competitive transplantation experiments, equal numbers of CD44-deficient or intact CD5+/CD19+ cells were used for staining with CellTrace Violet (CTV) or carboxyfluorescein succinimidyl ester (CFSE) Cell Proliferation Kit (Thermo Fisher). The ratio in the final mixture was determined again. For anti-CD44 treatment studies, splenocytes were incubated with anti-CD44 (clone KM201, 5 µg/mL) for 20 minutes at 37°C where indicated. After 3 hours or 3 days, mice were sacrificed, and the number of CD5+/CD19+ cells that had homed to BM, spleen, and PB was detected using CD5- and CD19-specific antibodies and CellTrace dye. Homing rate was calculated as described.12,15 Proliferation after 3 days was determined using CellTrace dye dilution.
CLL PBMCs were treated with anti-CD44 (515 Fab fragment, 5 µg/mL) for 10 minutes at 37°C where indicated. Human homing assays were previously described.12
Whole transcriptome analysis
Gene expression profiling of splenic B cells from Tcl1-tg, Cd44ΔB Tcl1-tg, C57BL/6J, and Cd44ΔB C57BL/6J mice was performed after sorting of tumor cells (CD5+/CD19+) or healthy B cells (CD5−/CD19+) from diseased and age-matched healthy animals of each genotype (4 mice each group). Purity after sorting was >95%. Clariom S assay (Affymetrix) was performed by the Center of Competence for Fluorescence Bioanalytic and Microarray Technology, Germany. Data were analyzed using the Transcriptome Analysis Console (Affymetrix).
Gene set enrichment analysis (GSEA)
Gene sets were manually curated from the MSigDB_v4.0 database (Broad Institute). GSEA was performed as previously described,16 considering gene signatures that obtained a significant (P < .05, false discovery rate [FDR] < 25%) enrichment score. Signatures with <10 genes or >500 genes were filtered out.
Cell culture
Splenocytes were cultured 24 to 48 hours with or without M2-10B4 stromal cells (ATCC-CRL-1972) and treated with 5 µg/mL anti-CD44v6 (clone 9A4) or anti-panCD44 (clone KM201) where indicated.
Human CLL PBMCs were cultured with or without Human T-Activator CD3/CD28 Dynabeads (Thermo Fisher) for 24 to 120 hours as described.2 In addition, CLL PBMCs were cultured with or without M2-10B4 stromal cells, or NIH3T3 fibroblasts transfected with CD40L or empty vector, kindly provided by Arnon Kater.17 Cells were treated with 10 µg/mL anti-immunoglobulin M (IgM) F(ab')2, 1 µM ibrutinib for 24 hours, or with 10 µg/mL high-molecular-weight HA for 10 minutes where indicated.
Western blotting
Isolated CD5+/CD19+ cells from CLL patients (purity >96%) were analyzed as described11 using primary antibodies against phosphorylated and unphosphorylated inhibitory NF-κB inhibitor (IkB), IκB kinase (IKK), and actin.
Inhibition assays
For NF-κB inhibition assays, PBMCs from CLL patients were preincubated for 30 minutes with 20 µM of the caspase inhibitor Q-VD-OPh and subsequently for 1 hour with 10 µM of the NF-κB inhibitor BAY-11-7082 (Sigma-Aldrich). Medium was removed; fresh medium was added, and cells were seeded on NIH3T3 fibroblasts with or without CD40L expression. For MEK inhibition, cells were treated with 2 or 10 µM Cobimetinib (APExBIO) for 24 hours.
Chromatin immunoprecipitation
Mec1 cells (ACC 497, DSMZ) were authenticated by DNA fingerprinting and passaged <6 months. Chromatin immunoprecipitation (ChIP) was performed as described using tumor necrosis factor-α (TNF-α)–stimulated Mec1 cells.11 Primers were designed based on in silico ENCODE-project data18 to validate the p65-binding region starting at position +128 relative to the transcriptional start site of CD44 (NM_001001392): primer: “p65 binding region fw”: 5′-GCAAATCCCAGCCCTGCTTTCC-3′; “p65 binding region rv”: 5′-CAAGATGGGTGCGGGGTGCT-3′.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5. Box plots are shown with whiskers from minimum to maximum. All data sets were tested for normal distribution using the Kolmogorov-Smirnov test. Outliers according to the Grubbs’ test were excluded from analysis. Two groups of normally distributed data were compared using the paired or unpaired Student t test and nonparametric data sets were analyzed for paired analysis with the Wilcoxon signed-rank tests or for unpaired analysis Mann-Whitney U test. Three or more groups of data were analyzed with the 1-way analysis of variance with post hoc tests. Results were considered significantly different when P < .05, with values at *P < .05, **P < .01, and ***P < .001. Nonsignificant differences were marked as ns.
Study approval
Blood samples were obtained upon written informed consent (Ethics committee Salzburg approval: 415-E/1287/4-2011, 415-E/1287/8-2011, 415-E/1287/13-2016) from CLL patients at the Third Medical Department, Paracelsus Medical University, Salzburg. Animal experimentation approval numbers are 20901-TVG/89/7-2014 and 20901-TVG/52/11-2012.
For the experiment procedures of the supplemental figures, see supplemental Methods.
Results
CD44 deficiency of murine B cells delays leukemic onset by modulating tumor infiltration of spleen
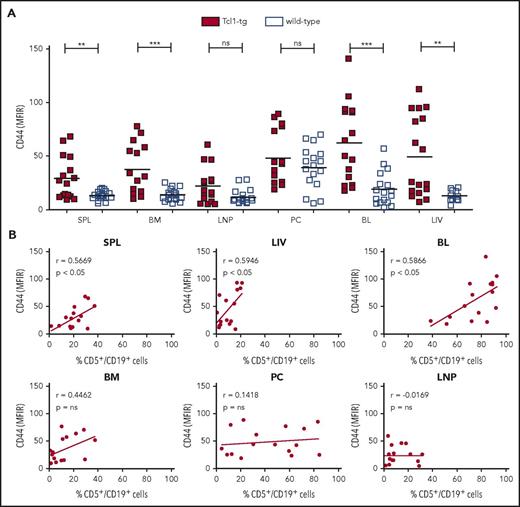
We used the Tcl1-tg model, mirroring human CLL to study the in vivo expression and function of CD44 in lymphoid organs. We first analyzed CD44 protein levels of CD5+/CD19+ cells derived from spleen, BM, lymph nodes, PC, blood, and liver of Tcl1-tg mice with overt leukemia by flow cytometry (Figure 1A). CD5+/CD19+ cells in all organs, except lymph nodes and PC, displayed significantly increased CD44 expression compared with normal B cells (CD5−/CD19+ cells; Figure 1A) or B1a cells (CD5+/CD19+; supplemental Figure 1) derived from wild-type animals. CD44 intensity correlated with the number of infiltrating leukemic cells in spleen, blood, and liver, but not in the other investigated organs (Figure 1B).
CD44 is overexpressed on leukemic cells of Tcl1-tg mice compared with healthy B cells of wild-type mice and is associated with organ infiltration. (A) CD44 surface expression on viable CD5+/CD19+ leukemic cells of Tcl1-tg mice and CD5−/CD19+ healthy B cells of C57BL/6J wild-type mice was assessed by flow cytometry (mean fluorescence intensity ratio, MFIR). (B) CD44 expression on CD5+/CD19+ cells in lymphoid organs of Tcl1-tg mice correlates with the percentage of CD5+/CD19+ CLL cell infiltration in spleen (SPL), liver (LIV), and blood (BL) but not BM, lymph node (LNP), and PC. Pearson correlation was determined.
CD44 is overexpressed on leukemic cells of Tcl1-tg mice compared with healthy B cells of wild-type mice and is associated with organ infiltration. (A) CD44 surface expression on viable CD5+/CD19+ leukemic cells of Tcl1-tg mice and CD5−/CD19+ healthy B cells of C57BL/6J wild-type mice was assessed by flow cytometry (mean fluorescence intensity ratio, MFIR). (B) CD44 expression on CD5+/CD19+ cells in lymphoid organs of Tcl1-tg mice correlates with the percentage of CD5+/CD19+ CLL cell infiltration in spleen (SPL), liver (LIV), and blood (BL) but not BM, lymph node (LNP), and PC. Pearson correlation was determined.
Next, by crossing Tcl1-tg mice with CD19Cre9 and Cd44flox/flox mice,10 we established a unique CLL mouse model with a B-cell–specific Cd44 knockout, CD19Cre/+ Cd44flox/flox Tcl1-tg mice, henceforth referred to as Cd44ΔB Tcl1-tg. This conditional model allowed us to dissect the contribution of CD44 expressed on leukemic cells without affecting CD44 in the microenvironment. CLL development in blood of Cd44ΔB Tcl1-tg mice compared with parental Tcl1-tg mice and to nonleukemic C57BL/6J wild-type mice was regularly assessed. Three months after birth, Tcl1-tg mice developed a population of leukemic CD5+/CD19+ B cells, whereas Cd44ΔB Tcl1-tg animals lacked leukemia. At the age of 5 months, the tumor load of Cd44ΔB compared with CD44 intact Tcl1-tg animals was still significantly reduced, but with increasing age, the mice developed normal leukemia (Figure 2A) with no significantly different survival rates (supplemental Figure 2A). The CD44 knockout was highly efficient and tightly restricted to the B-cell lineage in spleen, BM, lymph node, liver, PC, and blood of sacrificed moribund end-stage CLL mice (supplemental Figure 2B). CD44 expression on other cell types like T cells was normal (supplemental Figure 2C).
B-cell–specific CD44 deletion leads to a delayed leukemic onset and reduced tumor infiltration in vivo. (A) A CLL mouse model with a B-cell–specific Cd44 knockout, henceforth the Cd44ΔB Tcl1-tg mouse, was established. The amount of PB CD5+/CD19+ cells per microliter in wild-type, Tcl1-tg, and Cd44ΔB Tcl1-tg mice was measured by flow cytometry during early disease development (3 and 5 months) and progressive disease (7 and 9 months). (B) Organ infiltration of Tcl1-tg and Cd44ΔB Tcl1-tg mice at end stage of disease or age-matched C57BL/6 (wild-type) mice was determined by histology of 3 different mice per genotype. Hematoxylin and eosin staining of LIV, lung (LUG), SPL, and BM of 3 different mice of each genotype was analyzed. Images from representative regions are shown. (Ci) Representative spleens of Tcl1-tg, Cd44ΔB Tcl1-tg, and wild-type mice are shown. (Cii) Spleen size and weight were measured at end stage of disease from Tcl1-tg and Cd44ΔB Tcl1-tg mice. (D) The absolute number of CD5+/CD19+ cells in the whole spleen (i) and in both femora (ii) of end-stage diseased Tcl1-tg (n = 12) and Cd44ΔB Tcl1-tg (n = 14) mice was determined by flow cytometry. Mio, million.
B-cell–specific CD44 deletion leads to a delayed leukemic onset and reduced tumor infiltration in vivo. (A) A CLL mouse model with a B-cell–specific Cd44 knockout, henceforth the Cd44ΔB Tcl1-tg mouse, was established. The amount of PB CD5+/CD19+ cells per microliter in wild-type, Tcl1-tg, and Cd44ΔB Tcl1-tg mice was measured by flow cytometry during early disease development (3 and 5 months) and progressive disease (7 and 9 months). (B) Organ infiltration of Tcl1-tg and Cd44ΔB Tcl1-tg mice at end stage of disease or age-matched C57BL/6 (wild-type) mice was determined by histology of 3 different mice per genotype. Hematoxylin and eosin staining of LIV, lung (LUG), SPL, and BM of 3 different mice of each genotype was analyzed. Images from representative regions are shown. (Ci) Representative spleens of Tcl1-tg, Cd44ΔB Tcl1-tg, and wild-type mice are shown. (Cii) Spleen size and weight were measured at end stage of disease from Tcl1-tg and Cd44ΔB Tcl1-tg mice. (D) The absolute number of CD5+/CD19+ cells in the whole spleen (i) and in both femora (ii) of end-stage diseased Tcl1-tg (n = 12) and Cd44ΔB Tcl1-tg (n = 14) mice was determined by flow cytometry. Mio, million.
Histology of spleen, lungs, and liver at end-stage CLL (10-12 months) revealed residual intact structures of Cd44ΔB Tcl1-tg organs, whereas a strong leukemic infiltration disrupted the organ architecture in CD44-proficient mice. Unexpectedly, B-cell–specific CD44 depletion did not affect leukemic infiltration of BM (Figure 2B). Lower leukemic infiltration in spleens of Cd44ΔB Tcl1-tg mice was paralleled by decreased spleen size and weight (Figure 2C), confirmed by flow cytometric determination of the absolute CD5+/CD19+ cell count in spleen and BM (Figure 2D). Collectively, CD44 was not essential for accumulation of leukemic cells in BM but important for their accumulation in spleen, restricting initial phases of CLL.
CD44 is primarily required for early leukemic B-cell engraftment in spleen
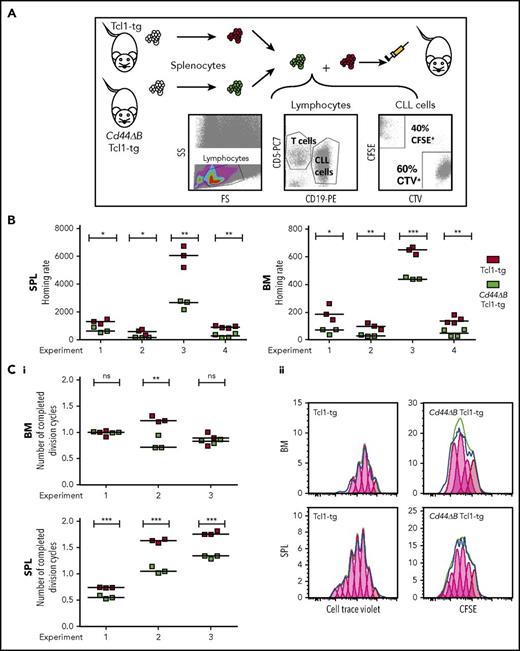
Having observed a spleen-specific but not BM-specific association of CD44 with the degree of leukemic infiltration, we next analyzed the homing and engraftment capacity of CD44-proficient and CD44Δ leukemic B cells by competitive adoptive transfers. Splenocytes of Cd44ΔB Tcl1-tg- and CD44-proficient Tcl1-tg animals were labeled with different proliferation dyes, mixed, and coinjected IV into wild-type syngeneic mice. Mice were sacrificed after 3 hours (short-term homing, allowing leukemia cell entry into organs but no proliferation) or 3 days posttransplantation (engraftment, which includes first proliferation events). Injected CD5+/CD19+ cells were cytometrically identified in spleen and BM (Figure 3A). CD44 intact leukemic B cells had a significantly greater capacity to home within 3 hours to both BM and spleen, compared with CD44ΔB leukemic B cells (Figure 3B). Within 3 days, CD44 intact leukemic B cells, compared with CD44ΔB cells, transversed the cell cycle in spleen more often, promoting early engraftment (Figure 3C). However, BM engraftment did not consistently depend on CD44, suggesting its selective role in CLL entry to BM rather than its proliferative capacity in this organ. Dye-swap control experiments confirmed these results (data not shown).
CD44 deficiency of leukemic B cells leads to a homing defect to SPL and BM and reduces proliferation of leukemic cells in SPL in competitive adoptive transfer experiments. (A) Scheme of short-term, competitive, adoptive transfer approach. Splenocytes of Tcl1-tg and Cd44ΔB Tcl1-tg mice were analyzed for the content of leukemic CD5+/CD19+ B cells and stained with different cell dyes, CFSE or CTV, mixed and injected into tail veins of 3 to 4 C57BL/6J recipients per experiment. After 3 hours or 3 days, the number of CD5+/CD19+ cells that had homed to the SPL and BM of the recipients was determined by flow cytometry. (B) Homing of CD5+/CD19+ cells after 3 hours was determined as previously described,11,13 in 4 independent experiments using splenocytes from 4 different Tcl1-tg and 4 Cd44ΔB Tcl1-tg mice. Briefly, homing rate is defined as the number of measured leukemic cells per 1 million measured lymphocytes per 1 million injected cells. (Ci) The number of completed division cycles of injected CD5+/CD19+ cells in SPL and BM of C57BL/6J wild-type recipients after 3 days was analyzed by cell trace dye dilution with FlowJo software and quantified in 3 independent experiments. (Cii) Representative histogram plots of CFSE and CTV fluorescence of in vivo proliferated CD5+/CD19+ cells in SPL and BM after 3 days in C57BL/6J wild-type mice are shown.
CD44 deficiency of leukemic B cells leads to a homing defect to SPL and BM and reduces proliferation of leukemic cells in SPL in competitive adoptive transfer experiments. (A) Scheme of short-term, competitive, adoptive transfer approach. Splenocytes of Tcl1-tg and Cd44ΔB Tcl1-tg mice were analyzed for the content of leukemic CD5+/CD19+ B cells and stained with different cell dyes, CFSE or CTV, mixed and injected into tail veins of 3 to 4 C57BL/6J recipients per experiment. After 3 hours or 3 days, the number of CD5+/CD19+ cells that had homed to the SPL and BM of the recipients was determined by flow cytometry. (B) Homing of CD5+/CD19+ cells after 3 hours was determined as previously described,11,13 in 4 independent experiments using splenocytes from 4 different Tcl1-tg and 4 Cd44ΔB Tcl1-tg mice. Briefly, homing rate is defined as the number of measured leukemic cells per 1 million measured lymphocytes per 1 million injected cells. (Ci) The number of completed division cycles of injected CD5+/CD19+ cells in SPL and BM of C57BL/6J wild-type recipients after 3 days was analyzed by cell trace dye dilution with FlowJo software and quantified in 3 independent experiments. (Cii) Representative histogram plots of CFSE and CTV fluorescence of in vivo proliferated CD5+/CD19+ cells in SPL and BM after 3 days in C57BL/6J wild-type mice are shown.
Consistent with the competitive transfers, treatment of CD44-proficient leukemic B cells by a blocking anti-CD44 antibody abrogated their homing capacity to spleen and BM (Figure 4A-B). Residual BM homing was still dependent on VLA-4 (CD49d/CD29) (supplemental Figure 3A), confirming our previous data.12 However, the CD49d expression on CD5+/CD19+ cells from Cd44ΔB Tcl1-tg was significantly decreased compared with CD5+/CD19+ cells from Tcl1-tg mice (supplemental Figure 3B).
CD44 blockade reduces homing of murine and human leukemic cells. (A) Scheme of short-term adoptive transfer approach. CTV-stained splenocytes of Tcl1-tg mice with anti-CD44 antibody (clone KM201) treatment or untreated controls (UTC) were injected into the tail veins of C57BL/6J recipients. Alternatively, human PBMCs from CLL patients with or without anti-CD44 antibody (clone 515) treatment were injected into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) recipients. After 3 hours, the number of CD5+/CD19+ cells that had homed to SPL and BM of the recipients was determined by flow cytometry using human-specific anti-CD45, anti-CD5, and anti-CD19 antibodies. (B) The rate of CD5+/CD19+ cells from Tcl1-tg mice that had homed to SPL and BM of the recipients was determined in 4 independent experiments. In each experiment, technical duplicates were performed. (C) The rate of CD5+/CD19+ cells from CLL patients that had homed to SPL and BM of the recipients was determined in 7 independent experiments using samples from 7 different CLL patients (all CD49d+, 4 MCLL, 3 UMCLL). In each experiment, technical duplicates were performed, and they were averaged for the analysis.
CD44 blockade reduces homing of murine and human leukemic cells. (A) Scheme of short-term adoptive transfer approach. CTV-stained splenocytes of Tcl1-tg mice with anti-CD44 antibody (clone KM201) treatment or untreated controls (UTC) were injected into the tail veins of C57BL/6J recipients. Alternatively, human PBMCs from CLL patients with or without anti-CD44 antibody (clone 515) treatment were injected into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) recipients. After 3 hours, the number of CD5+/CD19+ cells that had homed to SPL and BM of the recipients was determined by flow cytometry using human-specific anti-CD45, anti-CD5, and anti-CD19 antibodies. (B) The rate of CD5+/CD19+ cells from Tcl1-tg mice that had homed to SPL and BM of the recipients was determined in 4 independent experiments. In each experiment, technical duplicates were performed. (C) The rate of CD5+/CD19+ cells from CLL patients that had homed to SPL and BM of the recipients was determined in 7 independent experiments using samples from 7 different CLL patients (all CD49d+, 4 MCLL, 3 UMCLL). In each experiment, technical duplicates were performed, and they were averaged for the analysis.
To validate these observations in human CLL, we performed short-term adoptive transfers of human CD49d+ CLL PBMCs (4 patients with mutated IgVH genes [MCLL], 3 patients with unmutated IgVH genes [UMCLL] ) into immunodeficient mice, as previously established.12,15,19 Pretreatment of PBMCs with anti-CD44 Fab fragments (clone 515), reported to block CD44 binding of HA,20,21 significantly reduced CLL cell homing to spleen. BM homing was also reduced by CD44 blockage but not at statistical significance (P = .0778) (Figure 4C). Collectively, these results indicate that the murine spleen contains unique niches for CD44-mediated homing and survival of both murine and human CLL cells.
CD44-deficient leukemic cells harbor decreased NF-κB activation and proliferative disadvantages
We next determined expression of the activation marker CD86 and the proliferation marker Ki-67 of leukemic B cells derived from spleen and BM of Tcl1-tg and Cd44ΔB Tcl1-tg animals. CD44 deficiency was paralleled by significantly reduced CD86 expression (Figure 5Ai). Spleen-derived leukemic B cells, compared with those derived from BM, expressed significantly higher percentages of Ki-67+ cells. In spleen, Ki-67 expression was reduced when CD44 was depleted. In BM, Ki-67 expression was slightly, yet not significantly, different in Tcl1-tg and Cd44ΔB Tcl1-tg mice (Figure 5Aii). Using genome-wide transcriptomics, we examined relative changes in gene expression of splenic leukemic cells derived from diseased Tcl1-tg vs diseased Cd44ΔB Tcl1-tg mice, 4 mice, respectively. Figure 5B presents unsupervised hierarchical clustering of these transcriptomic data, with 35 differentially regulated genes (supplemental Table 4), applying a twofold change cutoff and P < .05, among them members of the MAPK and NF-κB pathways. In addition, subjecting the raw microarray data to GSEA identified transcriptional changes in gene sets of B-cell–specific NF-κB targets (Jain NFKB_Signaling set; Figure 5Ci), also in the context of proliferation (Hallmark_MYC_targets; Figure 5Cii). Notably, these alterations were specific for malignant cells, with only 9 genes altered in CD44 intact vs CD44ΔB spleen samples of C57BL/6J wild types (data not shown). Thus, the association between CD44 and NF-κB signaling is pronounced in malignant rather than normal B cells.
CD44-intact Tcl1-tg leukemic cells have a proliferative advantage in spleen that can be attributed to differential gene expression signatures (eg, NF-κB signaling) compared with CD44-deficient cells. (Ai) CD86 surface expression and (ii) intracellular Ki-67 expression of freshly isolated SPL- and BM-derived CD5+/CD19+ cells from diseased Tcl1-tg (n = 12) and Cd44ΔB Tcl1-tg (n = 12) were determined. (B) Sorted splenic CD5+/CD19+ of diseased Tcl1-tg and Cd44ΔB Tcl1-tg mice (n = 4 of each genotype) were used to perform Clariom-S microarray. Hierarchical clustering of differentially expressed genes was performed using Transcriptome Analysis Console (Affymetrix). A list of those genes with fold change and P value is available in supplemental Table 4. (Ci) Representative enrichment plots for NF-κB signaling (JAIN_NFKB_SIGNALING) (normalized enrichment score [NES] = 1.66, nominal P = .0 via 1000 permutations, FDR q value = 0.049), (ii) and MYC signaling (HALLMARK_MYC_TARGETS_V2) (NES = 1.62, nominal P = .03 via 1000 permutations, FDR q value = 0.046) are shown. Heatmaps for genes included in the core enrichments are depicted underneath.
CD44-intact Tcl1-tg leukemic cells have a proliferative advantage in spleen that can be attributed to differential gene expression signatures (eg, NF-κB signaling) compared with CD44-deficient cells. (Ai) CD86 surface expression and (ii) intracellular Ki-67 expression of freshly isolated SPL- and BM-derived CD5+/CD19+ cells from diseased Tcl1-tg (n = 12) and Cd44ΔB Tcl1-tg (n = 12) were determined. (B) Sorted splenic CD5+/CD19+ of diseased Tcl1-tg and Cd44ΔB Tcl1-tg mice (n = 4 of each genotype) were used to perform Clariom-S microarray. Hierarchical clustering of differentially expressed genes was performed using Transcriptome Analysis Console (Affymetrix). A list of those genes with fold change and P value is available in supplemental Table 4. (Ci) Representative enrichment plots for NF-κB signaling (JAIN_NFKB_SIGNALING) (normalized enrichment score [NES] = 1.66, nominal P = .0 via 1000 permutations, FDR q value = 0.049), (ii) and MYC signaling (HALLMARK_MYC_TARGETS_V2) (NES = 1.62, nominal P = .03 via 1000 permutations, FDR q value = 0.046) are shown. Heatmaps for genes included in the core enrichments are depicted underneath.
Spleen-derived leukemic cells express CD44v6 with high affinity to HA contributing to proliferation
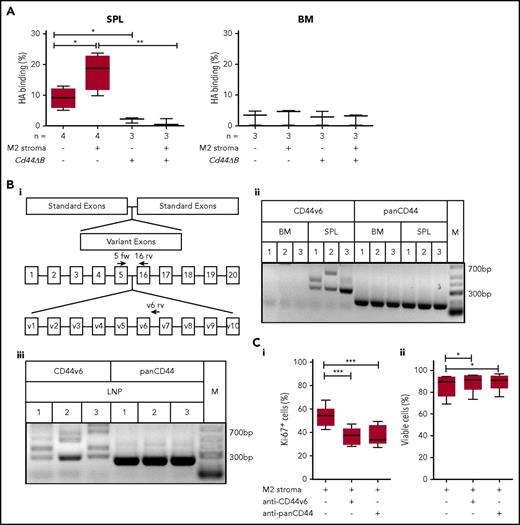
The spleen-specific CD44-dependent proliferation of leukemic B cells points to a differential CD44 ligand binding activity and isoform composition in this environment. We determined the HA binding capacity of leukemic B cells derived from Tcl1-tg spleen vs BM by flow cytometry using fluorescein-labeled HA. Spleen-derived leukemic B cells retained the capacity to bind HA ex vivo in a CD44-mediated manner. Stromal cell contact further increased this capacity. In contrast, BM-derived leukemic B cells displayed low affinity toward HA (Figure 6A). To elucidate the molecular basis for the differential affinity, we systematically examined the CD44v composition of leukemic B cells isolated from spleen and BM of Tcl1-tg mice with respect to variants 3, 6, 7, and 10, suggesting v6 as the major differentially regulated exon (data not shown). RT-PCR analysis confirmed the expression of long variants containing v6 in Tcl1-tg spleen but not BM, whereas CD44s expression was similar in both organs (Figure 6Bi-ii). Sequencing of bands confirmed CD44v6-containing isoforms, in variable combinations with v4 and v5 (supplemental Table 5). Notably, the lymph node microenvironment similarly to spleen induced v6-containing variants (Figure 6Biii; supplemental Table 5).
Spleen- but not BM-derived CLL cells bind HA and express long CD44 isoforms containing the variant exon 6, which is important for leukemic cell proliferation. (A) Cytometric analysis of HA binding of SPL- or BM-derived CD5+/CD19+ cells from Tcl1-tg and Cd44ΔB Tcl1-tg that were cultured with or without M2 stromal cells for 48 hours was performed. (Bi) Schematic diagram of the Cd44 gene and the primer design used for RT-PCR. Binding regions of the CD44s forward (5 fw) primer used in combination with a constant exon-specific (16 rv) or variant reverse primer (v6 rv) are indicated with arrows. (Bii) RT-PCR analysis of isoforms containing CD44v6 (5 fw and v6 rv primer combination) and panCD44 (5 fw and 16 rv primer combination) in the Cd44 cDNA from SPL- or BM-derived CD5+/CD19+ cells from Tcl1-tg mice (n = 3, indicated as 1-3) was performed (M1 spleen: lower band CD44v6, upper band CD44v5-6; M2 spleen: lower band CD44v6, upper band CD44v4-6; M3 spleen: CD44v6; supplemental Table 5). Low-range DNA ladder (M) in the right panel. (Biii) RT-PCR analysis in the Cd44 cDNA from LNP-derived CD5+/CD19+ cells from 3 Tcl1-tg mice (indicated as 1-3) (M1: lower band CD44v6, upper band CD44v3v6; M2: main band CD44v6; M3: lowest band CD44v6, second lowest band CD44v5-6; supplemental Table 5) (C) Intracellular Ki-67 expression (i) and percentage of viability (ii) of splenic CD5+/CD19+ leukemic cells cultured on M2 stromal cells and with or without anti-CD44v6 or anti-panCD44 antibody treatment of 48 hours was determined by flow cytometry (n = 6).
Spleen- but not BM-derived CLL cells bind HA and express long CD44 isoforms containing the variant exon 6, which is important for leukemic cell proliferation. (A) Cytometric analysis of HA binding of SPL- or BM-derived CD5+/CD19+ cells from Tcl1-tg and Cd44ΔB Tcl1-tg that were cultured with or without M2 stromal cells for 48 hours was performed. (Bi) Schematic diagram of the Cd44 gene and the primer design used for RT-PCR. Binding regions of the CD44s forward (5 fw) primer used in combination with a constant exon-specific (16 rv) or variant reverse primer (v6 rv) are indicated with arrows. (Bii) RT-PCR analysis of isoforms containing CD44v6 (5 fw and v6 rv primer combination) and panCD44 (5 fw and 16 rv primer combination) in the Cd44 cDNA from SPL- or BM-derived CD5+/CD19+ cells from Tcl1-tg mice (n = 3, indicated as 1-3) was performed (M1 spleen: lower band CD44v6, upper band CD44v5-6; M2 spleen: lower band CD44v6, upper band CD44v4-6; M3 spleen: CD44v6; supplemental Table 5). Low-range DNA ladder (M) in the right panel. (Biii) RT-PCR analysis in the Cd44 cDNA from LNP-derived CD5+/CD19+ cells from 3 Tcl1-tg mice (indicated as 1-3) (M1: lower band CD44v6, upper band CD44v3v6; M2: main band CD44v6; M3: lowest band CD44v6, second lowest band CD44v5-6; supplemental Table 5) (C) Intracellular Ki-67 expression (i) and percentage of viability (ii) of splenic CD5+/CD19+ leukemic cells cultured on M2 stromal cells and with or without anti-CD44v6 or anti-panCD44 antibody treatment of 48 hours was determined by flow cytometry (n = 6).
We next tested whether CD44v6 directly contributes to tumor proliferation using cocultures of Tcl1-tg splenocytes (containing leukemia cells and autologous T cells) with stromal cells. To that end, we analyzed the Ki-67 rates of the leukemic cells, identified by their CD5/CD19 coexpression. Treatment with anti-panCD44 and with a specific anti-CD44v6 antibody similarly impaired tumor cell proliferation, suggesting the dominant role of CD44v6 (Figure 6Ci, CLL cell viability; Figure 6Cii). This observation was further confirmed in a second set of experiments, counting absolute cell numbers (supplemental Figure 4A). Leukemic cell division upon Tcl1-tg splenocytes coculture with stromal cells was also monitored using CTV (supplemental Figure 4B).
NF-κB machineries in human CLL cells induce CD44v6 and support their proliferative capacity
We next validated our observations in human CLL. First, we screened 343 CLL blood samples for CD44v6. Only 4% (15 cases) of these mostly quiescent samples expressed basal CD44v6 expression, consistent with our previous observation.7 Notably, 14 of the 15 cases displayed unmutated IgVH genes, indicating active B-cell receptor (BCR) signaling22,23 linked to CD44v6. CD44v6 positivity was also associated with ZAP-70 expression, but not with CD38 and CD49d positivity (supplemental Figure 5A). We also found that within these samples, the CLL subset, that had recently proliferated and emigrated from lymphoid organs, defined by the bright CD5 (CD5bright) and diminished CXCR4 (CXCR4dim) expression signature,24 displayed further increased CD44v6 levels (Figure 7A). In line with this finding, CD44v6 and CXCR4 expression correlated negatively, and CD44v6 and CD5 expression correlated positively, both in a linear manner (supplemental Figure 5B).
NF-κB- and MAPK-induced CD44v6 expression is linked to human CLL cell proliferation. (A) CXCR4, CD5, and CD44v6 were costained on PBMCs from CD44v6+ CLL patients. (i) Cells were gated according to Calissano et al24 ; representative dot plot is shown. (ii) Mean fluorescence intensity (MFI) of CD44v6 was compared between the CXCR4highCD5low and the CXCR4lowCD5high subpopulation of CD5+/CD19+ cells (n = 8). (Bi) PBMCs from CLL patients were cultured with M2 stromal cells with or without anti-CD3/CD28 activating beads (T act) or NIH3T3 fibroblasts transfected with or without CD40L (3T40L) for 24 hours. CD44v6 surface expression was determined by flow cytometry (n = 4). (Bii) PBMCs from CLL patients were cultured with M2 stromal cells with or without anti-IgM antibody or ibrutinib for 24 hours. CD44v6 surface expression was determined by flow cytometry (n = 4). (C) PBMCs from CLL patients were cultured with NIH3T3 fibroblasts or 3T40L and treated with 2 or 10 µM cobimetinib for 24 hours. CD44v6 surface expression was determined by flow cytometry (n = 5). (Di) Protein lysates from isolated CLL cells (patients 1-3, PAT 1-3) that were cultured with NIH3T3 fibroblasts transfected with or without CD40L for 24 hours were tested for their IkB α, phospho-IkB α, IKK α (upper band), and phospho-IKK α (upper band) β (lower band) content by western blot. (Dii-iii) PBMCs from CLL patients were treated for 0.5 hour with pan-caspase inhibitor (Q-VD-OPh) and an NF-κB inhibitor (BAY11-7082) for 1 hour and then cultured with NIH3T3 fibroblasts transfected with or without CD40L for 24 hours. (ii) CD44v6 and (iii) CD69 surface expression on viable CD5+/CD19+ CLL cells was determined by flow cytometry (n = 8). (iv) p65 ChIP analysis of the Cd44 promoter was conducted using an NF-κB activated, CLL patient-derived cell line Mec-1 (bars, mean ± standard deviation). One representative out of 3 independent experiments, performed in duplicates, is shown. (E) Unstimulated PBMCs from CLL patients were cultured for 24 hours and treated with HA for 10 minutes. Phosphorylation of ERK was measured in CD5+/CD19+ CLL cells via flow cytometry (n = 5). (F) PBMCs from CLL patients were cultured with NIH3T3 fibroblasts transfected with or without CD40L for 24 hours. HA binding and phosphorylation of p65 (i) and ERK (ii) were measured via flow cytometry (n = 5). (G) PBMCs from CLL patients were cultured with NIH3T3 fibroblasts transfected with or without CD40L for 72 hours with or without anti-CD44v6 or anti-panCD44 antibody. Intracellular Ki-67 expression was determined by flow cytometry (n = 6).
NF-κB- and MAPK-induced CD44v6 expression is linked to human CLL cell proliferation. (A) CXCR4, CD5, and CD44v6 were costained on PBMCs from CD44v6+ CLL patients. (i) Cells were gated according to Calissano et al24 ; representative dot plot is shown. (ii) Mean fluorescence intensity (MFI) of CD44v6 was compared between the CXCR4highCD5low and the CXCR4lowCD5high subpopulation of CD5+/CD19+ cells (n = 8). (Bi) PBMCs from CLL patients were cultured with M2 stromal cells with or without anti-CD3/CD28 activating beads (T act) or NIH3T3 fibroblasts transfected with or without CD40L (3T40L) for 24 hours. CD44v6 surface expression was determined by flow cytometry (n = 4). (Bii) PBMCs from CLL patients were cultured with M2 stromal cells with or without anti-IgM antibody or ibrutinib for 24 hours. CD44v6 surface expression was determined by flow cytometry (n = 4). (C) PBMCs from CLL patients were cultured with NIH3T3 fibroblasts or 3T40L and treated with 2 or 10 µM cobimetinib for 24 hours. CD44v6 surface expression was determined by flow cytometry (n = 5). (Di) Protein lysates from isolated CLL cells (patients 1-3, PAT 1-3) that were cultured with NIH3T3 fibroblasts transfected with or without CD40L for 24 hours were tested for their IkB α, phospho-IkB α, IKK α (upper band), and phospho-IKK α (upper band) β (lower band) content by western blot. (Dii-iii) PBMCs from CLL patients were treated for 0.5 hour with pan-caspase inhibitor (Q-VD-OPh) and an NF-κB inhibitor (BAY11-7082) for 1 hour and then cultured with NIH3T3 fibroblasts transfected with or without CD40L for 24 hours. (ii) CD44v6 and (iii) CD69 surface expression on viable CD5+/CD19+ CLL cells was determined by flow cytometry (n = 8). (iv) p65 ChIP analysis of the Cd44 promoter was conducted using an NF-κB activated, CLL patient-derived cell line Mec-1 (bars, mean ± standard deviation). One representative out of 3 independent experiments, performed in duplicates, is shown. (E) Unstimulated PBMCs from CLL patients were cultured for 24 hours and treated with HA for 10 minutes. Phosphorylation of ERK was measured in CD5+/CD19+ CLL cells via flow cytometry (n = 5). (F) PBMCs from CLL patients were cultured with NIH3T3 fibroblasts transfected with or without CD40L for 24 hours. HA binding and phosphorylation of p65 (i) and ERK (ii) were measured via flow cytometry (n = 5). (G) PBMCs from CLL patients were cultured with NIH3T3 fibroblasts transfected with or without CD40L for 72 hours with or without anti-CD44v6 or anti-panCD44 antibody. Intracellular Ki-67 expression was determined by flow cytometry (n = 6).
We next incubated resting CD44v6-negative CLL cells with activated T cells or with CD40L-overexpressing fibroblasts, stimuli that promote CLL proliferation,2 thereby inducing de novo CD44v6 expression (Figure 7Bi). BCR stimulation by anti-IgM also resulted in CD44v6 induction, which was reversed by ibrutinib treatment (Figure 7Bii). Notably, in normal B cells, IgM and CD40L stimulation induced comparable CD44v6 induction and activation (supplemental Figure 5Ci-ii), suggesting CD44v6 modulation as a general immunological feature of B-cell activation.
These observations suggested an involvement of the MAPK and the NF-κB signaling pathways in the induction of CD44v6. We first used the MEK inhibitor cobimetinib as a tool to investigate the contribution of MAPK pathway to CD40L-induced CD44v6 expression. Indeed, cobimetinib inhibited CD44v6 induction in a dose-dependent manner (Figure 7C). Next, we tested active NF-κB signaling after CD40L stimulation, which revealed higher phospho–inhibitor of NF-κB subunit beta (IKKB) α and phospho-IkB α (Figure 7Di). Pretreatment of CLL cells with an NF-κB inhibitor before their stimulation with CD40L prevented the induction of CD44v6 expression (Figure 7Dii) and their activation (CD69; Figure 7Diii). To overcome toxicity of the NF-κB inhibition, an additional caspase inhibitor was used to block apoptosis (supplemental Figure 5D). Finally, to analyze whether the NF-κB subunit p65 directly binds the CD44 promoter, we performed ChIP assays using stimulated Mec1 cells, a CLL patient-derived cell line. ChIP with anti-p65 but not with control IgG resulted in robust enrichment of the CD44 promoter fragment containing the predicted NF-κB binding sites (Figure 7Div). These results collectively suggest that microenvironment-induced NF-κB signaling can directly regulate CD44 mRNA expression and selective splicing of CD44 variants.
Having elucidated the upstream modulation of CD44v6, we next investigated downstream effects of CD44v6 induction and function. HA treatment of unstimulated CLL cells with low CD44v6 expression (range 0.8% to 7.2% CD44v6) directly induced phosphorylation of extracellular signal-regulated kinase (ERK), as assessed by phospho flow cytometry (Figure 7E). Upon CD40L stimulation, phospho-ERK and phospho-p65 levels were further increased, in line with their increased CD44v6 expression, up to 60% (data not shown). Importantly, cells capable of binding HA had higher phospho-ERK and phospho-p65 levels (Figure 7F). Finally, both anti-CD44v6 and anti-panCD44 antibody treatment interfered with CD40L-stimulated CLL proliferation (Figure 7G), and blocking CD44v6 with an isoform-specific antibody25 reduced the division of the CLL subcohort with basal CD44v6 expression (supplemental Figure 6). Collectively, the data suggest a circuit of CLL proliferation, CD44v6 expression, and function, which involves the activation of NF-κB and MAPK pathways members both upstream and downstream of CD44v6.
Discussion
The functional activity of CD44 is tightly regulated by transcriptional splicing.6 Occurrence of CD44v6 has been attributed to malignancy,6 but its regulation and signaling are not well understood. Here, we established a conditional murine CLL model with a B-cell–specific CD44 knockout and provide evidence for the robust association of CD44v6 expression on spleen-derived CLL cells with accelerated proliferation and leukemic dissemination. We found substantial differences of the spleen and BM microenvironments regarding their ability to induce CD44v6 variants. We also describe a link between CD44v6 expression, MAPK signaling, and NF-κB machineries, shared by both murine and human CLL.
The Tcl1-tg mouse reproduces leukemia with a similar course to aggressive human CLL.26 In human CLL, tumor cells divide mainly in secondary lymphoid organs, where accessory cells (eg, stromal cells and T cells) provide the suitable environment to maintain proliferation and survival.24 In the Tcl1-tg CLL model, the spleen is the dominant proliferative compartment, where follicular dendritic cells within splenic B-cell follicles support CLL survival.27 Accordingly, we found the highest proliferation rates in the spleen of Tcl1-tg mice. Expression of CD44 on splenocytes was particularly relevant for early disease dynamics and leukemic dissemination by shaping homing, engraftment, and proliferation in this organ.
Investigating the underlying signaling pathways, we found that splenic B cells lacking CD44 display reduced NF-κB transcript signatures. This is consistent with the fact that the long-term maintenance of any mature B cell critically depends on NF-κB.28,29 Mature splenic B cells exhibit higher constitutive activity of NF-κB than many other leukocytes and, according to the name, NF-κB was discovered as a “nuclear factor ‘kappa-light-chain-enhancer’ of activated B cells.”30 Importantly, the basal activity of this pathway is further upregulated in tumors.31 Tcl1-tg mice recapitulate the human situation, with NF-κB cascades in lymphoid organs triggered by BCR signaling or microenvironmental signals, shaping CLL pathophysiology.32,33 We found that CD44v6 expression and HA-binding capacity are linked to active MAPK and NF-κB signaling, critical for CLL proliferation. Oppositely, we also observed that both pathways induce the active CD44 isoform CD44v6, proposing an upstream and downstream MAPK/NF-κB-CD44 circuit. This implies that therapies suppressing NF-κB signaling, as described for ibrutinib, for example,34 will result in reduced CD44v6 expression and function, and that CD44v6 could serve as a biomarker to monitor active disease under therapy.
Using a germline CD44 knockout model, Fedorchenko et al described that CD44 serves as a prosurvival factor during disease development of Tcl1-tg mice. 35 Several other groups reported on the contribution of CD44-mediated processes to cell survival in vitro by acting in part through a multiprotein complex comprising CD44, MMP9, and VLA-4.36-39 Our B-cell–specific model allows for the first time the differentiation between the contribution of CD44 expressed on CLL cells vs CD44 expressed by cells of the microenvironment. It revealed the transforming and proliferative nature of CD44v6 rather than CD44s expressed by the leukemic cells, which we propose to function during early disease onset, leukemic engraftment, and spreading. This contrasts the late and survival phenotype observed by Fedorchenko et al,35 which might also involve a contribution of CD44 expressed on accessory cells, such as T cells or macrophages.
Our data are consistent with the previous suggestion of CD44 as a leukemia stem cell molecule,5 and the association of CD44v6 variants with poor prognosis and metastasis in various tumors.40 However, we propose that particularly the switch between standard CD44 and CD44v6 modulates leukemia initiation and progressive disease. We also provide direct functional evidence that CD44v6-HA interactions support malignant B-cell proliferation, which is linked to the higher adhesive capacity of the tumor cells to their HA-bearing prosurvival stromal elements. Notably, CD44 deficiency in healthy B cells of C57BL/6J mice had far less impact on the whole transcriptome, consistent with previous observations using germline CD44 knockout models that displayed only minor or no phenotypes in respect to recirculation and differentiation of lymphocytes.41
Our data suggest CD44v6 as a marker for active disease in B-cell and other malignancies. A CD44v6-specific targeting may allow the reduction of described side effects of anti-panCD44 antibodies,42-45 which bind CD44 abundantly expressed by other cell types. In this regard, it is interesting that T cells that were engineered to target CD44v6 variants exert potent antitumor effects in both acute myeloid leukemia and multiple myeloma.46 CD44v6 targeting by peptides44 as well as newly engineered CD44v-specific chimeric antigen receptor T cells may be novel tools to treat hematological and other tumors.
Taken together, on basis of our in vivo murine model and our various ex vivo and in vitro approaches, we propose that MAPK and NF-κB–regulated CD44v6 variants on leukemic B cells with high affinity to HA rather than the standard CD44 isoform with low affinity to HA promote B-cell leukemia progression.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE109121).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the patients for their participation in this study. The authors also thank Christoph Ratswohl for helpful revision of the manuscript and Ursula Denk, Jan Höpner, and Mathias Hopfinger for experimental support.
This work was supported by the SCRI-LIMCR GmbH, the Province of Salzburg, Austrian Science Fund (FWF) 25015-B13 (T.N.H.), and the program Immunity in Cancer and Allergy (W1213, Austrian Science Fund) (T.N.H., F.A., and R.G.).
Authorship
Contribution: J.C.G. and T.N.H. oversaw the conception and design of this work; J.C.G., M.S., S.R., C.S., and T.G. developed the methodology; J.C.G., E.S., L.T., D.A., X.Y., and C.S. oversaw the acquisition of data; J.C.G., E.S., L.T., D.A., M.S., S.R., T.G., X.Y., V.O.-R., C.S., F.A., R.A., L.K., and T.N.H. analyzed and interpreted the data; J.C.G., R.A., and T.N.H. wrote the manuscript; J.C.G., E.S., L.T., D.A., M.S., S.R., X.Y., T.G., C.S., A.E., F.A., R.A., L.K., R.G., V.O.-R., and T.N.H. reviewed and/or revised the manuscript; R.G., V.O.-R., F.A., and T.N.H. provided administrative, technical, or material support; and T.N.H. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tanja N. Hartmann, Third Medical Department with Hematology, Oncology, Hemostaseology, Infectiology, and Rheumatology, Oncologic Center, Paracelsus Medical University, Salzburg, Austria; e-mail: t.hartmann@salk.at.




![Figure 5. CD44-intact Tcl1-tg leukemic cells have a proliferative advantage in spleen that can be attributed to differential gene expression signatures (eg, NF-κB signaling) compared with CD44-deficient cells. (Ai) CD86 surface expression and (ii) intracellular Ki-67 expression of freshly isolated SPL- and BM-derived CD5+/CD19+ cells from diseased Tcl1-tg (n = 12) and Cd44ΔB Tcl1-tg (n = 12) were determined. (B) Sorted splenic CD5+/CD19+ of diseased Tcl1-tg and Cd44ΔB Tcl1-tg mice (n = 4 of each genotype) were used to perform Clariom-S microarray. Hierarchical clustering of differentially expressed genes was performed using Transcriptome Analysis Console (Affymetrix). A list of those genes with fold change and P value is available in supplemental Table 4. (Ci) Representative enrichment plots for NF-κB signaling (JAIN_NFKB_SIGNALING) (normalized enrichment score [NES] = 1.66, nominal P = .0 via 1000 permutations, FDR q value = 0.049), (ii) and MYC signaling (HALLMARK_MYC_TARGETS_V2) (NES = 1.62, nominal P = .03 via 1000 permutations, FDR q value = 0.046) are shown. Heatmaps for genes included in the core enrichments are depicted underneath.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/12/10.1182_blood-2017-08-802462/4/m_blood802462f5.jpeg?Expires=1767767073&Signature=A4snuj4p1hGg8Cj5AOxF~-H4mwcgkkrNQkVf-rTT41I0UBEw6JcNxYnM5-ZKQeeVjDIhsafaK8uEhBjZVcR350cSvOqZt~1pP0~PeV8Mxo-yLtFwuUhaZozbbyCv5aZVr250nndkYrlwizJpHOhcxNd~LHK~zUWfMGJrKf3PEhmlkILXoubiZibZkLKhM~B1ne0BVc97eWFfmBPnWQt8ytGRyjFPoYOQRIzVjeW7dEcQ80oZorne5k6qajB6k4NONhmElCCWUzMNJvS4RRJAne2nx9SDPI3YgXZwfr-gmon-ibHhNyn4JWx8bOwDhbqK8iW48TgXMxRXzMnHInHDmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal