Fibrosis constitutes the end stage of the inflammatory process in chronic graft-versus-host disease (GVHD) leading to major morbidity in affected patients. In this issue of Blood, Yamakawa and coworkers provide compelling evidence that vitamin A–coupled liposomes carrying heat shock protein 47 (HSP47) small interfering RNA (siRNA) ameliorate chronic GVHD-induced fibrosis.1
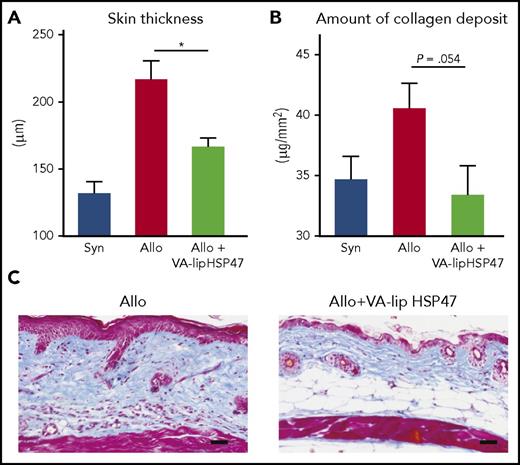
VA-lip HSP47 resolves established skin fibrosis in chronic GVHD. Skin sickness in allogeneic recipients as compared with allogeneic recipients treated with VA-lip HSP47 (A). This reduced sickness is related due to reduced amount of collagen deposit in the skin of treated mice (B). This direct effect of VA-lip HSP47 on collagen deposit is illustrated by the 2 skin biopsies (C) of nontreated and VA-lip HSP47-treated recipients. Allo, allogeneic; Syn, syngeneic; VA-lip HSP47, vitamin A–coupled liposomes carrying HSP47 siRNA. The figure has been adapted from Figure 4 in the article by Yamakawa et al that begins on page 1476.
VA-lip HSP47 resolves established skin fibrosis in chronic GVHD. Skin sickness in allogeneic recipients as compared with allogeneic recipients treated with VA-lip HSP47 (A). This reduced sickness is related due to reduced amount of collagen deposit in the skin of treated mice (B). This direct effect of VA-lip HSP47 on collagen deposit is illustrated by the 2 skin biopsies (C) of nontreated and VA-lip HSP47-treated recipients. Allo, allogeneic; Syn, syngeneic; VA-lip HSP47, vitamin A–coupled liposomes carrying HSP47 siRNA. The figure has been adapted from Figure 4 in the article by Yamakawa et al that begins on page 1476.
Chronic GVHD is the leading cause of late morbidity and late nonrelapsed mortality after allogeneic hematopoietic stem cell transplantation. Chronic GVHD manifests with multiorgan pathology with the skin, mucosa, and lung being the most commonly involved target organs. Pathologists have described 4 different stages: (1) epidermotropic T-cell infiltration and target cell phase, (2) the lichenoid phase, (3) inflammatory fibrosis phase, and (4) the sclerodermatous phase. Thus, fibrosis may function as a wound-healing process in some chronic inflammatory processes. Unfortunately, in chronic GHVD, fibrosis leads to chronic disability when it affects joints or extensive skin areas. Fibrosis may lead to severe life-threatening chronic respiratory deficiency if it involves the peribronchiolar pulmonary lobules.2-4 Once fixed (and nonevolutive), fibrosis is poorly amenable to any therapeutic measure.
The pathways leading to fibrosis have only been recently dissected through experimental transplantation models. As recently reviewed by Zeiser and Blazar,3 the last phase of tissue inflammation in chronic GHVD is characterized by aberrant tissue repair promoted by activated macrophages. Geoffrey Hill’s group nicely demonstrated that activated macrophages produce transforming growth factor-β (TGF-β) and platelet-derived growth factor-α leading to fibroblast activation. Activated fibroblasts produce procollagen, then matrix collagen, that ultimately leads to tissue stiffness.5 In some models, the chronic inflammatory state is maintained by T-helper 17 cells.2-4
In this issue of Blood, Yamakawa et al designed an elegant set of experiments showing that targeting HSP47, a collagen-specific molecular chaperone that plays a critical role in collagen synthesis in myofibroblasts, leads to an impressive reduction in collagen deposition without inducing systemic immunosuppression (see figure). They first showed using immunofluorescent staining an accumulation of myofibroblasts in the dermis of animals after allogeneic transplantation. These myofibroblasts coexpressed HSP47, and retinol-binding protein 1 (RBP1), a cellular retinol binding protein. Then, they reported that colony-stimulating factor 1 (CSF1) receptor-dependent macrophages play a critical role in accumulation of HSP47+ myofibroblasts in the chronic GVHD skin. Using vitamin A–coupled liposomes (that bind RBP1) carrying HSP47 siRNA (to inhibit HSP47),6 they demonstrated that they can inhibit HSP47 expression in TGF-β-stimulated fibroblasts in vitro and that VA-lip HSP47 specifically target skin fibrotic lesion in a multiple minor mismatched chronic GVHD model. The antifibrotic effects of VA-lip HSP47 was not strain dependent, because VA-lip HSP47 abrogated accumulation of HSP47+ myofibroblasts and ameliorated skin fibrosis in another major mismatched mouse model of cutaneous chronic GVHD where mice were transplanted with granulocyte colony-stimulating factor mobilized splenocytes.
These results are consistent with Hill’s study demonstrating that depletion of macrophages by an anti-CSF1-receptor monoclonal antibody markedly reduces GVHD pathology.5 The role of HSP47 in fibrosis is further supported by increased HSP47 expression in the kidneys of patients with renal allograft failure7 and in lacrimal glands of patients with chronic GVHD.8
In the article, the authors also reported that VA-lip injected IV selectively targeted skin fibrotic lesions (without affecting normal skin), suggesting that fibroblasts residing in the normal skin might express fewer vitamin A receptors than pathogenic myofibroblasts.
VA-lip HSP47 is now under clinical trials in human liver cirrhosis (https://clinicaltrials.gov/ct2/results?cond=&term=ND-L02). Because VA-lip HSP47 does not seem to suppress T-cell responses, it may represent a novel therapeutic tool in addition to standard immunosuppressive GVHD treatment of patients with chronic GVHD. However, vitamin A has a pleiotropic role, particularly in regulating mucosal immunity.9 Vitamin A has a profound effect on antigen uptake by epithelial cells; it influences antigen processing and presentation and effects mucosal homing of T and B cells. It will thus be important to evaluate if VA-lip HSP47 has any effects on vitamin A receptor expressing cells other than myofibroblasts.
Pirfenidone, a US Food and Drug Administration–approved antifibrotic agent against idiopathic lung fibrosis, has also been reported recently to ameliorate experimental chronic GVHD in the lung and skin via inhibition of macrophage infiltration and TGF-β production.10
In conclusion, both pirfenidone and VA-lip HSP47 show promise in the prevention and treatment of GVHD-induced fibrosis. Given the unmet clinical need of targeting this end-stage process and its debilitating consequences in the daily life of the patients with chronic GVHD, these studies raise hope of moving the field from the bench to the bedside.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal