Key Points
GDF-15 level is a new prognostic factor for survival in AL amyloidosis, and its reduction after therapy is associated with better outcome.
GDF-15 level is probably the strongest predictor for renal outcomes in patients with AL amyloidosis.
Abstract
Growth differentiation factor-15 (GDF-15) improves prognostication in patients with cardiovascular disorders in addition to conventional cardiac markers (N-terminal pro B-type natriuretic peptide [NT-proBNP], troponins [Tns]) and has shown prognostic value in patients with renal diseases. In patients with light chain (AL) amyloidosis, cardiac involvement is the major determinant of prognosis, and cardiac markers define prognosis, whereas biomarkers of renal involvement stratify renal risk. We explored the prognostic importance of serum level of GDF-15 in patients with AL amyloidosis in 2 independent cohorts. The prognostic value of GDF-15 level was initially evaluated in a cohort of 107 consecutive previously untreated patients with AL amyloidosis from Athens, Greece, and was then validated in a second cohort of 202 consecutive previously untreated patients from Pavia, Italy. High GDF-15 level was associated with a higher risk of early death and poor overall survival independently of NT-proBNP and high-sensitivity TnT (hsTnT) or hsTnI levels. At the 6-month landmark, reduction of GDF-15 level ≥25% was associated with improved outcome. GDF-15 level ≥4000 pg/mL was associated with a high risk of progression to dialysis, independently of renal risk defined by estimated glomerular filtration rate and proteinuria, in both cohorts; failure to reduce GDF-15 below this level was associated with increased risk at either the 3- or 6-month landmark, independently of the established renal response or progression criteria. In conclusion, GDF-15 has prognostic implications for different outcomes in patients with AL and adds prognostic information independent of that provided by cardiac and renal risk biomarkers.
Introduction
Cardiac biomarkers have been the most powerful tool to define prognosis in amyloid light chain (AL) amyloidosis and are used to assess severity of cardiac dysfunction and assist in treatment decisions.1-4 A major advantage of cardiac biomarkers is their widespread availability, easy measurement, and reproducibility. Similar to cardiac biomarkers, renal biomarkers have also been used to develop a risk stratification system regarding the risk of progression to dialysis.5 However, accurate prognosis for the identification of patients at high risk of early death or those at high risk of progression to dialysis within intermediate-risk groups of patients with AL amyloidosis is challenging.
Growth differentiation factor-15 (GDF-15; also called macrophage inhibitory cytokine-1 [MIC-1]) has been identified as a biomarker that may improve prognostication and assessment of response to therapy in patients with cardiovascular diseases.6 GDF-15 is a member of the transforming growth factor β family and is involved in several pathological conditions, including inflammation, cancer, and cardiovascular, pulmonary, and renal diseases.7 Cardiac myocytes produce and secrete GDF-15 in response to oxidative stress, stimulation with angiotensin II or proinflammatory cytokines, ischemia, and mechanical stretch.8-11 Macrophages, vascular smooth muscle cells, endothelial cells, and adipocytes also produce GDF-15 in response to oxidative or metabolic stress or stimulation with proinflammatory cytokines.12-16 GDF-15 is induced in hypertrophic and dilated cardiomyopathy after volume overload, ischemia, and heart failure.8,17 GDF-15 has prognostic value in patients with cardiovascular disorders, adding prognostic information to that of conventional prognostic factors, such as N-terminal pro B-type natriuretic peptide (NT-proBNP) and high-sensitivity troponin T (hs-TnT).18-25 Furthermore, studies in patients with diabetes or other types of chronic renal diseases have indicated that higher levels of GDF-15 are associated with faster deterioration of kidney function and progression to end-stage renal disease (ESRD).26-31
Thus, we evaluated GDF-15 in 2 independent cohorts of patients with AL amyloidosis as a potential prognostic factor for early mortality, overall survival (OS), and renal outcomes and as a potential marker of response to therapy, in parallel with the assessment of contemporary and established cardiac and renal biomarkers.
Patients and methods
Patients
The analysis included consecutive unselected patients with newly diagnosed, previously untreated AL amyloidosis with available baseline serum samples (ie, before initiation of therapy). These patients were treated in the Department of Clinical Therapeutics (Athens, Greece) or the Amyloidosis Research and Treatment Center (Pavia, Italy). Patients provided written informed consent for blood sampling and access to their medical data for the purposes of this study. An approval for data publication was obtained by the local ethics committees/scientific review boards; clinical and response data were collected prospectively in all patients, and all patients were assessed and followed rigorously according to prespecified institutional protocols and received similar supportive care according to the practice of each institution. Standard criteria were used for the definition of organ involvement and assessment of response.32,33
Measurement of GDF-15 and cardiac biomarkers
We measured the circulating levels of GDF-15, NT-proBNP, and hs-TnT or troponin I (TnI) in the frozen sera of patients with newly diagnosed AL amyloidosis, before and at 3 and 6 months after treatment initiation. GDF-15 was measured by a novel immunoassay, the Elecsys GDF-15 assay (provided free of charge by Roche Diagnostics International Ltd, Rotkreuz, Switzerland). The Elecsys GDF-15 assay is an electrochemiluminescence immunoassay for use on cobas e immunoassay analyzers. The assay is based on the sandwich immunoassay principle and uses biotin-streptavidin technology. Samples (35 μL) were incubated for 9 minutes with a biotinylated monoclonal anti–GDF-15 antibody and a second monoclonal anti–GDF-15 antibody labeled with a ruthenium complex [Tris(2,2′-bipyridyl)ruthenium(II)] to form a sandwich complex when GDF-15 was present. Streptavidin-coated microparticles were added in a second 9-minute incubation period. Unbound substances were subsequently removed using a ProCell washing solution, and antigen-antibody complexes were detected via electrochemiluminescence using cobas e analyzers. Results were determined via an instrument-specific calibration curve generated by 2-point calibration and a master curve provided via the reagent barcode. The assay is reported to have coefficients of variation for repeatability and intermediate precision ranging from 0.7% to 1.5% and 2.4% to 3.1%, respectively, for samples containing 460 to 18 690 pg/mL, with a linear measuring range up to 20 000 pg/mL and limit of quantitation of 400 pg/mL.34 Measurements were performed at the Department of Clinical Biochemistry, “Aghia Sophia” Children’s Hospital, and clinical biochemists were blinded to clinical data. Roche Diagnostics provided the immunoassay free of charge and had no interference in the design of the study, serum measurements, analysis or interpretation of the results, or manuscript preparation. No other financial support was provided by Roche.
Statistical analysis
The cutoffs for GDF-15 levels were first identified in the cohort of patients who were treated in Athens (n = 107 patients) and then validated in the cohort of patients from Pavia (n = 202 patients). The cutoff levels were defined by using a receiver operating characteristic (ROC) analysis with death at 3 months and dialysis at 24 months as binary variables, similar to previous reports of biomarker evaluation in AL amyloidosis.5 For comparisons of differences among groups, the χ2 test was used for categorical variables (using Fisher’s exact test when appropriate) and the Mann-Whitney test or analysis of variance for continuous variables. OS was calculated from the date of initiation of therapy until the date of last follow-up or death. Survival curves were plotted with the Kaplan-Meier method, and comparisons were made using the log-rank test. For time-to-dialysis curves, death before dialysis was treated as a competing event.35,36 Multivariate analysis was performed using logistic regression and Cox proportional hazards. R software (http://www.r-project.org/) and SPSS 20 (IBM SPSS Statistics for Windows [version 20.0], Armonk, NY) were used.
Results
Table 1 lists the characteristics of the patients in the 2 cohorts. Median GDF-15 level was 3854 pg/mL (range, 626-71 475 pg/mL) in the Athens and 3027 pg/mL in the Pavia cohort (range, 624 to >100 000 pg/mL; P = .09). Thus, 90% and 94% of patients in the 2 cohorts had GDF-15 levels >1200 pg/mL (the upper limit of normal for individuals without cardiovascular disease; supplemental Figure 1, available on the Blood Web site).
Characteristics of patients in the study
| . | Athens (n = 107) . | Pavia (n = 202) . | P . |
|---|---|---|---|
| Male/female | 59%/41% | 54%/46% | .442 |
| Median age, years | 67 | 65 | .597 |
| dFLC >180 mg/L | 52% | 48.5% | .567 |
| Heart involvement | 62% | 77% | .007 |
| Mayo stage 1/2/3A/3B | 26%/43%/11%/15% | 15%/43%/27%/15% | .041 |
| Renal involvement | 72% | 67% | .415 |
| Median eGFR, mL/min per 1.73 m2 | 61 | 62 | .807 |
| eGFR <50 ml/min per 1.73 m2 | 34% | 36% | .545 |
| Median proteinuria, g/d | 6.5 | 5.1 | .544 |
| Proteinuria >5 g/d* | 61% | 56% | .178 |
| Renal stage 1/2/3† | 20%/54%/26% | 26%/54%/20% | .528 |
| Liver involvement | 17% | 12% | .284 |
| Nerve involvement | 25% | 11% | .001 |
| Primary therapy | <.001 | ||
| Bortezomib | 60% | 86% | |
| Lenalidomide | 33% | 0 | |
| MDex | 7% | 11% | |
| High-dose therapy with ASCT during disease course | 5% | 7% | .605 |
| CR + VGPR as response to primary therapy | 42% | 45% | .599 |
| . | Athens (n = 107) . | Pavia (n = 202) . | P . |
|---|---|---|---|
| Male/female | 59%/41% | 54%/46% | .442 |
| Median age, years | 67 | 65 | .597 |
| dFLC >180 mg/L | 52% | 48.5% | .567 |
| Heart involvement | 62% | 77% | .007 |
| Mayo stage 1/2/3A/3B | 26%/43%/11%/15% | 15%/43%/27%/15% | .041 |
| Renal involvement | 72% | 67% | .415 |
| Median eGFR, mL/min per 1.73 m2 | 61 | 62 | .807 |
| eGFR <50 ml/min per 1.73 m2 | 34% | 36% | .545 |
| Median proteinuria, g/d | 6.5 | 5.1 | .544 |
| Proteinuria >5 g/d* | 61% | 56% | .178 |
| Renal stage 1/2/3† | 20%/54%/26% | 26%/54%/20% | .528 |
| Liver involvement | 17% | 12% | .284 |
| Nerve involvement | 25% | 11% | .001 |
| Primary therapy | <.001 | ||
| Bortezomib | 60% | 86% | |
| Lenalidomide | 33% | 0 | |
| MDex | 7% | 11% | |
| High-dose therapy with ASCT during disease course | 5% | 7% | .605 |
| CR + VGPR as response to primary therapy | 42% | 45% | .599 |
ASCT, autologous stem-cell transplantation; CR, complete response; dFLC, difference between involved and uninvolved free light chains; eGFR, estimated glomerular filtration rate; MDex, melphalan plus dexamethasone; VGPR, very good PR.
In both cohorts, there was a strong correlation of GDF-15 with NT-proBNP (R2 = 0.211 and 0.124 in the 2 cohorts; P < .001 for both), Tns (hsTnT: R2 = 0.451 and P < .001; TnI: R2 = 0.332 and P < .001), and Mayo stage (analysis of variance P = .001; supplemental Figure 2) and an inverse correlation with eGFR (P < .001; supplemental Figure 3) but not with the amount of proteinuria. There was no statistically significant difference in the level of GDF-15 between men and women or in patients with or without renal, nerve, liver, or soft tissue involvement; there was also no correlation of GDF-15 level with the level of FLCs or with the extent of bone marrow plasma cell infiltration.37
GDF-15 in the test cohort
After a median follow-up of 4 years, 55% of the patients had died, and median survival was 47 months. In the univariate analysis, increasing GDF-15 level was associated with inferior survival (P = .011). To identify a cutoff level that could be used in clinical practice for evaluation of prognosis, we performed an ROC analysis with death at 3 months as a binary variable, and we identified GDF-15 level >7575 pg/mL (area under the curve, 0.77; 95% confidence interval [CI], 0.65-0.83; P = .004) as a predictor of early death; 3-month mortality was 40%, 12-month mortality was 65% (Figure 1A), and median OS was 6 months (vs 51 months for lower GDF-15 level; P = .003). In the multivariate analysis that included both NT-proBNP and hs-TnT or Mayo stage (stage 1, 2, 3A, or 3B), GDF-15 remained an independent prognostic factor for survival (hazard ratio [HR], 1.9; 95% CI, 1.1-3.9; P = .045; (Table 2; supplemental Tables 1 and 2).
Cumulative (cum) survival of patients from 2 cohorts. (A) Survival of patients with GDF-15 level >7575 pg/mL in the test cohort (Athens) and validation cohort (Pavia). (B) Landmark analysis at 6 months in patients who had a reduction of GDF-15 by ≥25% vs those without a decrease or with an increase in GDF-15 in both cohorts: in the Athens cohort (C) and the Pavia cohort (D).
Cumulative (cum) survival of patients from 2 cohorts. (A) Survival of patients with GDF-15 level >7575 pg/mL in the test cohort (Athens) and validation cohort (Pavia). (B) Landmark analysis at 6 months in patients who had a reduction of GDF-15 by ≥25% vs those without a decrease or with an increase in GDF-15 in both cohorts: in the Athens cohort (C) and the Pavia cohort (D).
Multivariate analysis for GDF-15 and survival in the 2 cohorts using cutoff values identified in initial testing cohort
| . | Athens . | Pavia . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Mayo stage | ||||||
| 1 | 1 | 1 | ||||
| 2 | 5.4 | 1.9-14.8 | .001 | 1.7 | 0.56-5.4 | .153 |
| 3A | 6.3 | 2-20 | <.001 | 2.1 | 0.8-7.5 | .108 |
| 3B | 9.6 | 3.1-29 | <.001 | 6.7 | 2.2-20.6 | <.001 |
| dFLC ≥180 mg/L | 1.15 | 0.67-1.9 | .734 | 2.5 | 1.4-4.6 | .01 |
| GDF−15 ≧7575 pg/mL | 2.0 | 1.1-3.6 | .023 | 1.92 | 1.1-3.35 | .022 |
| . | Athens . | Pavia . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Mayo stage | ||||||
| 1 | 1 | 1 | ||||
| 2 | 5.4 | 1.9-14.8 | .001 | 1.7 | 0.56-5.4 | .153 |
| 3A | 6.3 | 2-20 | <.001 | 2.1 | 0.8-7.5 | .108 |
| 3B | 9.6 | 3.1-29 | <.001 | 6.7 | 2.2-20.6 | <.001 |
| dFLC ≥180 mg/L | 1.15 | 0.67-1.9 | .734 | 2.5 | 1.4-4.6 | .01 |
| GDF−15 ≧7575 pg/mL | 2.0 | 1.1-3.6 | .023 | 1.92 | 1.1-3.35 | .022 |
Because GDF-15 level can reflect heart and renal dysfunction, we further evaluated whether GDF-15 could be associated with risk of progression to ESRD and need for dialysis. We analyzed patients with renal involvement and excluded patients requiring dialysis at the time of initiation of therapy; death resulting from cardiac causes was treated as a competing event for dialysis.
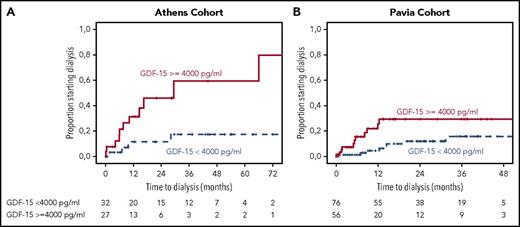
Using ROC analysis for requirement of dialysis at 2 years from start of therapy, GDF-15 level >4000 pg/mL was associated with a significant risk of progression to dialysis, with an associated HR of 6 (95% CI, 2-15.6; P = .001) and corresponding rates of progression to dialysis within 2 years of 7% vs 47% (Figure 2A). Following the staging system proposed by Palladini et al, eGFR <50 mL/min/m2 (P = .001) and proteinuria >5 g/d (P = .07) were also associated with higher risk of progression to dialysis in univariate analysis, and the relevant progression to dialysis rates for the 3 renal stages were 0%, 25%, and 47% at 2 years (P = .034). However, in multivariate analysis, which included GDF-15 >4000 pg/mL, eGFR <50 mL/min per 1.73 m2, and proteinuria >5 g/d, only GDF-15 was independently associated with a higher risk of ESRD (HR, 4; 95% CI, 1.16-13; P = .045); neither renal stage nor any of the variables comprising renal staging reached statistical significance.
Time to dialysis for patients from 2 cohorts. Time to dialysis for patients with renal involvement and GDF-15 level ≥4000 or <4000 pg/mL in the test cohort (A) and validation cohort (B), with cardiovascular death before dialysis as a competing event.
Time to dialysis for patients from 2 cohorts. Time to dialysis for patients with renal involvement and GDF-15 level ≥4000 or <4000 pg/mL in the test cohort (A) and validation cohort (B), with cardiovascular death before dialysis as a competing event.
GDF-15 in the evaluation cohort
We then evaluated the performance of GDF-15 in the testing cohort to patients from the Pavia cohort. Median follow-up of the Pavia cohort was 2 years; 2-year survival of patients in the Pavia cohort was 75% for those with GDF-15 <7575 pg/mL vs 48% for those with higher levels (P = .002; Figure 1B). We performed a multivariate analysis, which included GDF-15 <7575 pg/mL, Mayo stage, and difference between involved and uninvolved FLC level; GDF-15 was independently associated with survival (HR, 1.92; 95% CI, 1.1-3.35; P = .022; Table 2). We further evaluated the prognostic performance of GDF-15 in the validation cohort using C-statistic. The C-statistic for Mayo stage alone was 0.62 (95% CI, 0.54-0.7); it was 0.60 (95% CI, 0.53-0.68) for GDF-15 alone. The C-statistic for a combined model of Mayo stage and GDF-15 above the defined cutoff was 0.72 (95% CI, 0.63-0.81), and this difference was statistically significant (P = .01).
Regarding renal outcomes in the Pavia cohort, the 2-year dialysis rate was 37% for those with GDF-15 >4000 pg/mL vs 10% for those with lower levels (P = .004; Figure 2). In multivariate analysis, which also included renal staging, only GDF-15 level was associated with risk of progression to ESRD and dialysis (HR, 3; 95% CI, 1.6-15; P = .004; Table 3).
Baseline biomarkers associated with renal outcomes and landmark analysis for renal response, renal disease progression, and GDF-15 at 3 and 6 months
| . | Athens . | Pavia . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Renal stage | ||||||
| 1 | 1 | 1 | ||||
| 2 | 1.913 | 0.216-55 | .746 | 0.998 | 0.303-3.33 | .354 |
| 3 | 1.940 | 0.199-47 | .701 | 2.106 | 0.56-7.96 | .273 |
| GDF−15 >4000 pg/mL | 3.996 | 1.16-13.8 | .028 | 2.537 | 1.07-6.62 | .047 |
| . | Athens . | Pavia . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Renal stage | ||||||
| 1 | 1 | 1 | ||||
| 2 | 1.913 | 0.216-55 | .746 | 0.998 | 0.303-3.33 | .354 |
| 3 | 1.940 | 0.199-47 | .701 | 2.106 | 0.56-7.96 | .273 |
| GDF−15 >4000 pg/mL | 3.996 | 1.16-13.8 | .028 | 2.537 | 1.07-6.62 | .047 |
We further evaluated the prognostic performance of GDF-15 and renal staging in the validation cohort by means of the C-statistic, which was 0.59 (95% CI, 0.51-0.66) for renal stage alone and 0.71 (95% CI, 0.64-0.77) for GDF-15 ≥4000 pg/mL alone. The C-statistic for a combined model of renal stage and GDF-15 was 0.73 (95% CI, 0.64-0.88), and this difference was not statistically significant (P = .3).
Thus, we confirmed in an independent cohort of patients with AL amyloidosis that baseline GDF-15 level has prognostic significance for OS and renal outcomes, independent of that of other established prognostic factors.
Clinical significance of changes in GDF-15 level
At 3 months, among evaluable patients, 41% in the Athens cohort (median, −29%; range, −92% to −2%) and 26% in the Pavia cohort (median, −19%; range, −88% to −2%) had GDF-15 reduction. At the same time point, NT-proBNP response (≥30% reduction by ≥300 pg/mL) was achieved by 13% of evaluable patients in the Athens cohort and 21% of evaluable patients in the Pavia cohort, and eGFR was reduced by ≥25% in 36% and 25% of patients in the 2 cohorts, respectively. At 3 months, a hematologic response was achieved by 72% of patients in Athens cohort (≥ very good partial response [PR], 29%; PR, 43%) and 83% of patients in Pavia cohort (≥ very good PR, 39%; PR, 44%). In both cohorts, there was an association of hematologic response with GDF-15 reduction ≥25% (P = .094 and P = .023, respectively). At the 6-month landmark, there was a correlation of the reduction of GDF-15 level with NT-proBNP level reduction (P = .012 in Athens and P < .001 in Pavia cohort), and a ≥25% decrease of GDF-15 was associated with improved survival in both cohorts (Figure 1B), so in the Athens cohort (n = 76 evaluable patients), GDF-15 responders had a 2-year OS of 91% vs 66% (P = .03), and in the Pavia cohort (n = 110 evaluable patients), the 2-year survival rates were 100% vs 84% (P = .047). We then pooled patients from the 2 cohorts to evaluate GDF-15 response according to NT-proBNP response groups; patients who achieved both NT-proBNP response and GDF-15 reduction ≥25% had better outcome (2-year survival, 100% vs 82%; P = .044). Among patients without NT-proBNP response at 6 months, those with a GDF-15 reduction had improved outcome (2-year survival, 91% vs 74%; P = .12).
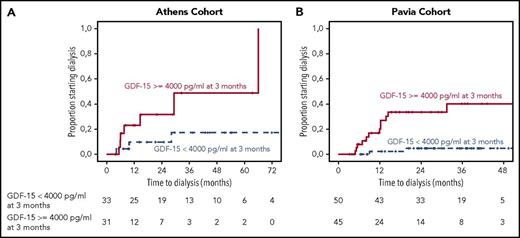
We then investigated the importance of serum GDF-15 changes on renal outcomes. At 3 months, 23% and 20% of patients with baseline GDF-15 ≥4000 pg/mL in the 2 cohorts had reduced their GDF-15 to <4000 pg/mL; failure to reduce GDF-15 to <4000 pg/mL or an increase of GDF-15 to ≥4000 pg/mL (52% and 48% of patients in the 2 cohorts, respectively) was associated with a 2-year progression rate to dialysis rate of 48% vs 10% in the Athens cohort (P = .01) and 37% vs 3% in the Pavia cohort (P < .001). At the same time point, 34% of patients in the Athens cohort and 27% in the Pavia cohort had renal progression (ie, ≥25% decrease in eGFR), and 25% and 19% had renal response (ie, ≥30% reduction in proteinuria without decrease in eGFR), respectively. In multivariate analysis, failure to reduce GDF-15 to <4000 pg/mL or an increase of GDF-15 to >4000 pg/mL was the only factor associated with time to dialysis; neither renal progression by eGFR or renal response by proteinuria decrease had independent prognostic significance (supplemental Tables 3-7).
Similarly, at the 6-month landmark, 33% of patients in the Athens cohort and 24% in the Pavia cohort had renal progression (ie, ≥25% decrease in eGFR), and 41% and 36% had renal response, respectively, whereas 43.5% and 46% had GDF-15 ≥4000 pg/mL; 46% in the Athens cohort and 57% in the Pavia cohort with GDF-15 level ≥4000 pg/mL experienced progression to dialysis (P = .05 and P = .048, respectively; Figure 3). In multivariate analysis, GDF-15 that remained or increased to ≥4000 pg/mL and renal progression by eGFR were the only factors associated with ESRD (supplemental Tables 3-7). Importantly, among patients with renal progression by eGFR at 6 months, none of the patients who had sustained or decreased GDF-15 <4000 pg/mL experienced progression to dialysis (supplemental Figure 4).
Landmark analysis at 3 months for patients from the 2 cohorts. Landmark analysis for patients with renal involvement who reduced or sustained their GDF-15 level <4000 pg/mL and those who had an increase to ≥4000 pg/mL or did not reduce level to <4000 pg/mL in the test cohort (A) and validation cohort (B). Figures depict time to initiation of dialysis.
Landmark analysis at 3 months for patients from the 2 cohorts. Landmark analysis for patients with renal involvement who reduced or sustained their GDF-15 level <4000 pg/mL and those who had an increase to ≥4000 pg/mL or did not reduce level to <4000 pg/mL in the test cohort (A) and validation cohort (B). Figures depict time to initiation of dialysis.
Discussion
In the current study, we have confirmed in 2 independent cohorts of patients with AL amyloidosis that GDF-15 serum level could be a new biomarker that is associated with higher risk of early death, shorter OS, and higher probability of progression to dialysis. Furthermore, GDF-15 reduction during therapy was associated with better survival and renal outcomes. These results were independent of the levels of established cardiobiomarkers; thus, GDF-15 may be an additional marker that could improve prognostic evaluation and assessment of response to therapy in patients with AL amyloidosis. The measurement of serum GDF-15 is now readily available and has been integrated into clinical practice in cardiovascular38,39 and renal diseases.31 This new biomarker should also be viewed in the context of novel treatment approaches in AL amyloidosis, which include the clinical development of monoclonal antibodies targeting amyloid fibrils.
GDF-15 levels were above the upper limit for individuals without apparent cardiovascular disease19,34,40 in 90% to 95% of patients with AL amyloidosis, even among patients without cardiac involvement. However, GDF-15 is not a specific cardiac marker but rather a marker of systemic response, because it is not secreted only by cardiomyocytes but also by other cell types, even within the bone marrow microenvironment,37,41 endothelial cells,42 and macrophages,12 in response to various stress stimuli, especially those related to reactive oxygen species (ROS).7 Thus, a high level of GDF-15 may reflect not only the degree of cardiac stress but also a systemic proinflammatory condition. This proinflammatory condition and ROS could be related to the direct effects of toxic amyloidogenic light chains or to the systemic inflammatory state that occurs in patients with chronic heart failure.43 Monoclonal light chains in AL amyloidosis affect multiple organ systems in ways that are not only related to the deposition of the amyloid fibrils but also result from direct induction of proteotoxic stress.44 Preclinical models have shown that toxic light chains may directly cause dysfunction of cardiac myocytes,44-47 induced by the induction of ROS through pathways such as p38MAPK,45,46 resulting in cellular dysfunction and apoptosis. Monoclonal toxic light chains may also be directly toxic to other cell types, including mesangial48 and endothelial cells.49 Thus, GDF-15 may be a marker of systemic FLC-related proteotoxic stress. In this setting, GDF-15, as a biomarker reflecting cardiac and extracardiac abnormalities, integrates information from cardiac and extracardiac disease pathways in AL amyloidosis and provides incremental prognostic information, which can be added to that provided by Tn, NT-proBNP, and FLC levels.
Our data also show that GDF-15 may be a valuable new biomarker for the assessment of renal risk and renal response in patients with AL amyloidosis. Not only did GDF-15 level add prognostic information to the established renal risk stratification system, based on eGFR and proteinuria,5 but level ≥4000 pg/mL was the most important prognostic factor for dialysis (Figure 3). Furthermore, failure to reduce GDF-15 early (ie, at either 3 or 6 months) was a strong risk factor for progression to dialysis, stronger than the renal progression and response criteria at the same time points, in both cohorts. An explanation for this finding could be that cells located in the kidneys (endothelial, mesenchymal, or even tubular) may produce GDF-1526,27,30 in response to the toxic effects of amyloid fibrils or in response to proteotoxic stress caused by FLCs.48 A recent study showed that circulating GDF-15 level correlates with intrarenal GDF-15 messenger RNA transcript level, and increasing circulating GDF-15 level was independently associated with adverse renal outcome.31
A biomarker is even more useful when it can be applied in observing response to therapy and guiding treatment decisions. Reduction of GDF-15 level was associated with a more favorable outcome in terms of OS at the 6-month landmark and with more favorable renal prognosis at the 3- and 6-month landmarks. There has been extensive experience with the use of other cardiac biomarkers (NT-proBNP or BNP) as surrogates of organ response in patients with cardiac involvement early in the course of disease,33 and these remain powerful tools for response assessment and prognostication. The prognostic significance of GDF-15 level was validated in an independent cohort using the cutoff values identified in a test cohort. Importantly, the results from the Athens cohort were validated in the Pavia cohort, although there were some differences in the incidence of cardiac and nerve involvement and the type of primary therapy between the 2 cohorts. This strategy supports the prognostic importance of this biomarker; however, it does not necessarily identify the optimal cutoffs. Furthermore, given the progress in the clinical development of targeted therapies for AL amyloidosis, GDF-15 must be further evaluated in the setting of antifibril monoclonal antibodies. Because GDF-15 is produced by macrophages, the level could be increased if the macrophages are activated in response to a monoclonal antibody; however, this hypothesis has to be confirmed. In contrast, if such an increase were to occur in response to a monoclonal antibody, GDF-15 could be used as a marker of immune response to amyloid.
In conclusion, GDF-15 is a novel biomarker with prognostic implications, associated with a higher risk of early death and OS; importantly, it was the strongest prognostic factor for renal outcomes in 2 independent cohorts of patients with AL amyloidosis. GDF-15 adds prognostic information independent of that of traditional cardiac and renal biomarkers.
Presented in part at the 58th Annual Meeting of the American Society of Hematology, San Diego, CA, 5 December 2016, and 22nd Congress of the European Hematology Association, Madrid, Spain, 23 June 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
Assays for GDF-15, NT-proBNP, and hsTnT were provided by Roche free of charge.
Authorship
Contribution: E.K., M.A.D., G.M., and G.P. designed the study, analyzed and interpreted data, and wrote the manuscript; I.P. set up the GDF-15 measurements, designed the study, performed the laboratory analyses, interpreted data, and reviewed and edited the manuscript; A.A. with F.A. performed the laboratory analyses; E.E.-P., P.M., E.T., M.B., F.R., E. Psimenou, M.R., M.G., D.F., D.C.Z., E. Papadopoulou, and C.P. collected and analyzed the data and critically reviewed the manuscript.
Conflict-of-interest disclosure: A.A. is an employee of Roche Greece; E.K. has received honoraria from Amgen, Janssen, Genesis Pharma, Takeda, and Prothena; G.P. has honoraria from Celgene, Prothena, and Janssen-Cilag and serves on the advisory board for Janssen-Cilag; M.A.D. has honoraria from Amgen, Celgene, Janssen, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Efstathios Kastritis, Department of Clinical Therapeutics, National and Kapodistrian University of Athens, School of Medicine, 80 Vas.Sofias Ave, Athens, Greece, 11528; e-mail: ekastritis@gmail.com.