TO THE EDITOR:
Favism, or “favic crisis,” is a potentially life-threatening acute hemolysis elicited in carriers of low-activity glucose 6-phosphate dehydrogenase (G6PD) variants by ingestion of raw faba bean (Vicia faba L) (FB) seeds.1,2 In 2 surveys of hemolytic crises because of favism in Sardinia in 948 children aged 2 to 12 years and 610 adults, hemolysis was elicited by raw beans in 94.4% and 97% of all cases, respectively.3,4 Low-activity G6PD variants are the Mediterranean variant (1% to 5% residual activity)2,5 predominantly present in Sardinia and common in Mediterranean and Middle East countries and in the Indian subcontinent,5 and other G6PD variants common in China and the Far East.6 FBs are rich in proteins, starch, fibers, vitamins, and minerals and are low in fat.7 FB seeds are uniquely rich (5-14 g/kg wet weight) with the β-glucosides vicine (V) and convicine (C),7,8 which generate the redox-active aglycones divicine (D; 2,6-diamino-4,5-dihydroxypyrimidine) and isouramil (I; 6-amino-2,4,5-trihydroxypyrimidine)1,9 upon hydrolysis by the β-glucosidase very active in raw FBs.10,11 D/I share an identical mechanism of action and are considered to be causative elements of favism.9-11 In isolated, oxygenated red blood cells (RBCs), D/I generate semiquinoid free radicals12 that produce oxygen radicals and hydrogen peroxide,13 which rapidly oxidize reduced NAD phosphate (NADPH), reduced glutathione (GSH), thiol groups, and membrane lipids of RBCs10,11,14 ; transform oxy-hemoglobin in ferrylhemoglobin, methemoglobin, and hemichromes11,15-17 ; and release iron from hemoglobin and ferritin.14 Although in D/I-treated G6PD-normal RBCs GSH and NADPH are rapidly regenerated and oxidative modifications reversed, no reversal occurs in D/I-treated G6PD-deficient RBCs.10,11 Without the protective action of GSH and NADPH, a chain of oxidative events may enhance phagocytic removal of damaged RBCs.10,11 Indeed, RBCs isolated during hemolytic crisis have shown early decrease of GSH and NADPH10,11 ; peroxidation of membrane lipids14 ; increase of hemichromes, aggregated band 3,11,14-16 and intracellular calcium17 ; occurrence of rigid “cross-bonded RBCs”11 ; and increased phagocytic susceptibility.10,11,17
Balance studies between disappearance of RBCs and appearance of free hemoglobin in plasma and urine during hemolytic crisis10,11 and evidence of massive erythrophagocytosis at crisis apex18 have indicated that RBCs were largely removed by extravascular hemolysis. A minor share of RBCs break down by intravascular lysis resulting in occasional hemoglobinuria.2,4,10
A spontaneous mutant allele of vc gene in V. faba that induces very low levels of V/C has been isolated from a Greek FB cultivar (line 1268),19 leading to breeding of the low-V/C Divine cultivar (vc-) with 0.16 g/kg wet weight V/C, compared with high-V/C beans used here with 4.75 g/kg wet weight V/C.19
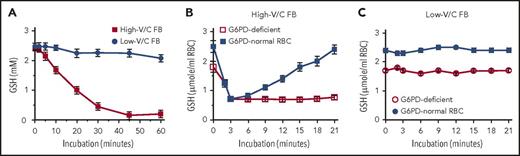
We show here that ingestion of large amounts of raw low-V/C FBs (Divine cultivar) by hemizygous and heterozygous carriers of low-activity G6PD (presumably G6PD-Mediterranean variant) did not cause oxidative RBC damage and hemolysis. In preliminary in vitro and ex vivo experiments, β-glucosidase–treated extracts from high- and low-V/C raw FBs were used to determine the potency to oxidize GSH in vitro and to challenge RBCs from G6PD-normal and G6PD-deficient subjects ex vivo (Figure 1). In vitro, in contrast to complete oxidation of GSH by high-V/C FB extracts, oxidation by low-V/C FB extracts was marginal (Figure 1A). In experiments with freshly isolated RBCs, high-V/C FB extracts decreased GSH level reversibly in G6PD-normal RBCs and irreversibly in G6PD-deficient RBCs (Figure 1B), whereas extracts from low-V/C FBs did not modify GSH levels either in G6PD-normal or G6PD-deficient RBCs (Figure 1C). These results were instrumental to obtain ethical clearance for in vivo testing of low-V/C FBs in G6PD-deficient subjects. Study protocol was approved by Alghero Hospital ethical committee (according to the Declaration of Helsinki), and informed consent was provided by all G6PD-normal and G6PD-deficient subjects (healthy adult hemizygous males and heterozygous females) of Sardinian ancestry. Hemizygous males were identified as G6PD deficient by quantitative assay of G6PD activity (<3% of normal activity). Heterozygous females (0.5% to 37.2% of normal activity) were sisters/daughters of G6PD-deficient males. None of the G6PD-deficient subjects had ever eaten FBs in any form. However, 1 or more close relatives (father, brother, uncle) had favism at child age. G6PD-normal controls were healthy adult subjects who had previously consumed high-V/C FBs without any adverse reaction. G6PD-activitiy and hematological data of all normal and G6PD-deficient subjects are shown in supplemental Tables 1-3 (available on the Blood Web site) and summarized in supplemental Figure 1. Three G6PD-deficient subjects, 2 women (B.M. and S.R.P.) and 1 man (D.F.G.) (see supplemental Tables 1-3) were silent carriers of β-thalassemia.
Effect of extracts from high- or low-V/C FBs on the GSH levels in a cell-free system and in isolated G6PD-normal and G6PD-deficient RBCs. Fresh FBs with high-V/C (average content of V/C in raw seeds: 4.75 g/kg wet weight) and with low-V/C (Divine cultivar, average content of V/C in raw seeds: 0.16 g/kg wet weight), were grown and collected at Institut National de la Recherche Agronomique, Dijon, France. FB seeds were immediately flash-frozen after harvest and kept on dry ice until usage. Thirty minutes before use, FBs were thawed, dehulled, suspended in degassed, nitrogen-flushed isotonic phosphate-buffered saline (PBS) at 50% weight-to-volume ratio, and homogenized in the cold in a nitrogen atmosphere with a high-speed GVA2-type homogenizer (Krups GmbH, Offenbach am Main, Germany). The slurry was centrifuged for 10 minutes at 15 000g and 4°C, and V/C assayed in the resulting extracts as indicated19 with pure vicine (Serva, Heidelberg, Germany) as a standard. Before starting incubations, the FB extracts were treated with 5 mg/mL β-glucosidase from almonds (Sigma, St. Louis, MO) during 60 minutes at 37°C to enhance the generation of the active compounds D and I from the inactive V/C. (A) Effect of high-V/C and low-V/C FB extracts on GSH oxidation in a cell-free system. GSH was dissolved at 2.5 mM (final concentration) in PBS-glucose, and FB extract added. The final concentration of V/C in the test system was 2.5 mM or 0.1 mM for high- and low-V/C, respectively. In control samples, PBS-glucose was added instead of the FB extract. The test solutions were incubated at 37°C. The GSH concentration was assessed time dependently as indicated.23 Mean values ±standard deviation (SD) of 3 independent experiments. (B) Effect of high-V/C FB extracts on GSH oxidation in isolated RBCs from G6PD-normal and G6PD-deficient subjects. Washed RBCs freshly isolated from hemizygous G6PD-deficient (open squares) and G6PD-normal (filled squares) subjects were resuspended in PBS-glucose at a hematocrit of 50%, supplemented at time 0 with extracts from high-V/C FBs at 2.5 mM V/C (final concentration) and incubated at 37°C. The GSH concentration in RBCs was assessed time dependently as indicated.23 Mean values ± SD of 3 independent experiments from 3 different subjects. (C) Effect of low-V/C FB extracts on GSH oxidation in isolated RBCs from G6PD-normal and G6PD-deficient subjects. Washed RBCs freshly isolated from hemizygous G6PD-deficient (open circles) and G6PD-normal (filled circles) subjects were resuspended in PBS-glucose at a hematocrit of 50%, supplemented at time 0 with extracts from low-V/C FBs at 0.1 mM V/C (final concentration), and incubated at 37°C. The GSH concentration in RBCs was assessed time dependently as indicated.23 Mean values ± SD of 3 independent experiments from 3 different subjects.
Effect of extracts from high- or low-V/C FBs on the GSH levels in a cell-free system and in isolated G6PD-normal and G6PD-deficient RBCs. Fresh FBs with high-V/C (average content of V/C in raw seeds: 4.75 g/kg wet weight) and with low-V/C (Divine cultivar, average content of V/C in raw seeds: 0.16 g/kg wet weight), were grown and collected at Institut National de la Recherche Agronomique, Dijon, France. FB seeds were immediately flash-frozen after harvest and kept on dry ice until usage. Thirty minutes before use, FBs were thawed, dehulled, suspended in degassed, nitrogen-flushed isotonic phosphate-buffered saline (PBS) at 50% weight-to-volume ratio, and homogenized in the cold in a nitrogen atmosphere with a high-speed GVA2-type homogenizer (Krups GmbH, Offenbach am Main, Germany). The slurry was centrifuged for 10 minutes at 15 000g and 4°C, and V/C assayed in the resulting extracts as indicated19 with pure vicine (Serva, Heidelberg, Germany) as a standard. Before starting incubations, the FB extracts were treated with 5 mg/mL β-glucosidase from almonds (Sigma, St. Louis, MO) during 60 minutes at 37°C to enhance the generation of the active compounds D and I from the inactive V/C. (A) Effect of high-V/C and low-V/C FB extracts on GSH oxidation in a cell-free system. GSH was dissolved at 2.5 mM (final concentration) in PBS-glucose, and FB extract added. The final concentration of V/C in the test system was 2.5 mM or 0.1 mM for high- and low-V/C, respectively. In control samples, PBS-glucose was added instead of the FB extract. The test solutions were incubated at 37°C. The GSH concentration was assessed time dependently as indicated.23 Mean values ±standard deviation (SD) of 3 independent experiments. (B) Effect of high-V/C FB extracts on GSH oxidation in isolated RBCs from G6PD-normal and G6PD-deficient subjects. Washed RBCs freshly isolated from hemizygous G6PD-deficient (open squares) and G6PD-normal (filled squares) subjects were resuspended in PBS-glucose at a hematocrit of 50%, supplemented at time 0 with extracts from high-V/C FBs at 2.5 mM V/C (final concentration) and incubated at 37°C. The GSH concentration in RBCs was assessed time dependently as indicated.23 Mean values ± SD of 3 independent experiments from 3 different subjects. (C) Effect of low-V/C FB extracts on GSH oxidation in isolated RBCs from G6PD-normal and G6PD-deficient subjects. Washed RBCs freshly isolated from hemizygous G6PD-deficient (open circles) and G6PD-normal (filled circles) subjects were resuspended in PBS-glucose at a hematocrit of 50%, supplemented at time 0 with extracts from low-V/C FBs at 0.1 mM V/C (final concentration), and incubated at 37°C. The GSH concentration in RBCs was assessed time dependently as indicated.23 Mean values ± SD of 3 independent experiments from 3 different subjects.
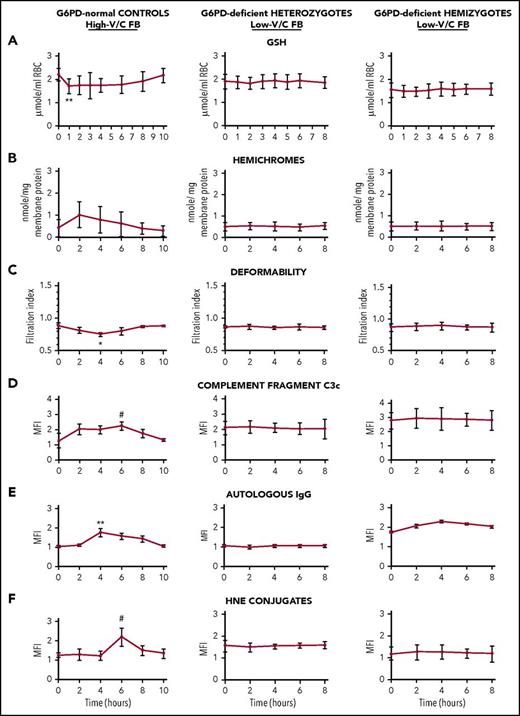
Selected early and sensitive indicators of oxidative damage prodromal of extravascular and intravascular hemolysis were analyzed time dependently in hemizygous and heterozygous G6PD-deficient subjects after ingestion of 500 g low-V/C raw FB seeds per 70 kg body weight (ie, 5-10 times the amount of an average FB meal). G6PD-normal subjects ingested the same amount of high-V/C raw FBs. As shown in Figure 2, in G6PD-normal subjects, some sensitive early indicators of RBC oxidative damage were transitorily altered, showing a short-term reversible decrease in GSH levels and RBC deformability, and increase in membrane-bound hemichromes and 4-hydroxynonenal (a lipoperoxidation product that triggers erythrophagocytosis when conjugated to membrane proteins20 ), in membrane-bound complement C3c fragments and autologous immunoglobulin. Modifications were generally modest, although occasionally significant, but fully reversible. Baseline levels were attained in all parameters after 8 to 10 hours from FB ingestion. By contrast, none of indicators of RBC oxidative damage and prodromal signals for phagocytic removal were significantly modified by low-V/C FB meal in any of hemizygous or heterozygous G6PD-deficient subjects (Figure 2). None of hemolysis indicators (ie, number of RBCs, hematocrit, RBC hemoglobin, serum haptoglobin, and indirect bilirubin) were significantly modified at any time during 2 days follow-up after low-V/C FB meal either in G6PD-normal or in G6PD-deficient study participants (supplemental Tables 1-3 and supplemental Figure 1). In sum, data shown in Figure 2, supplemental Table 1-3, and supplemental Figure 1 indicate that no RBC damage or intravascular hemolysis was taking place in any G6PD-deficient subject after consumption of low-V/C FBs. In normal subjects, none of the tested parameters were modified after ingestion of low-V/C FBs (not shown).
Time-dependent behavior of indicators of RBC oxidative damage in G6PD-normal subjects, G6PD-deficient heterozygous females, and G6PD-deficient hemizygous males after ingestion of high-V/C FBs (normal subjects) and low-V/C FBs (G6PD-deficient subjects). G6PD-normal controls (left panels; N = 9), G6PD-deficient heterozygous subjects (central panels, N = 7), and hemizygous subjects (right panels, N = 7) ingested 500 g/70 kg body weight freshly homogenized raw dehulled FBs at time 0. The G6PD-normal subjects ingested high-V/C FBs (average content of V/C in raw seeds: 4.75 g/kg wet weight) and low-V/C FBs (Divine cultivar, average content of V/C in raw seeds: 0.16 g/kg wet weight); the heterozygous and hemizygous G6PD-deficient subjects ingested low-V/C FBs. Blood was drawn via a cubital vein catheter within the Alghero Hospital setting under constant medical supervision immediately before the FB meal (time 0) and during the whole duration of the study as indicated. Indicators of RBC oxidative damage were measured time dependently as indicated: (A) intracellular GSH23 ; (B) RBC deformability by RBC filtration24 ; (C) membrane-bound hemichromes by luminescence-based heme quantification24 ; (D) membrane bound 4-hydroxynonenal (HNE) conjugates by fluorescence-activated cell sorting (FACS)25 ; (E) membrane-bound complement fragments C3c by FACS24 ; (F) membrane-bound autologous immunoglobulin G (IgG) by FACS.24 Mean values ± SD of 9 or 7 subjects as indicated. Data analyzed by Student t test. Symbols (*, #) indicate significantly different from time 0 value.**P < .005; *P < .01; #P < .05. For hematological data, see supplemental Tables 1-3 and supplemental Figure 1.
Time-dependent behavior of indicators of RBC oxidative damage in G6PD-normal subjects, G6PD-deficient heterozygous females, and G6PD-deficient hemizygous males after ingestion of high-V/C FBs (normal subjects) and low-V/C FBs (G6PD-deficient subjects). G6PD-normal controls (left panels; N = 9), G6PD-deficient heterozygous subjects (central panels, N = 7), and hemizygous subjects (right panels, N = 7) ingested 500 g/70 kg body weight freshly homogenized raw dehulled FBs at time 0. The G6PD-normal subjects ingested high-V/C FBs (average content of V/C in raw seeds: 4.75 g/kg wet weight) and low-V/C FBs (Divine cultivar, average content of V/C in raw seeds: 0.16 g/kg wet weight); the heterozygous and hemizygous G6PD-deficient subjects ingested low-V/C FBs. Blood was drawn via a cubital vein catheter within the Alghero Hospital setting under constant medical supervision immediately before the FB meal (time 0) and during the whole duration of the study as indicated. Indicators of RBC oxidative damage were measured time dependently as indicated: (A) intracellular GSH23 ; (B) RBC deformability by RBC filtration24 ; (C) membrane-bound hemichromes by luminescence-based heme quantification24 ; (D) membrane bound 4-hydroxynonenal (HNE) conjugates by fluorescence-activated cell sorting (FACS)25 ; (E) membrane-bound complement fragments C3c by FACS24 ; (F) membrane-bound autologous immunoglobulin G (IgG) by FACS.24 Mean values ± SD of 9 or 7 subjects as indicated. Data analyzed by Student t test. Symbols (*, #) indicate significantly different from time 0 value.**P < .005; *P < .01; #P < .05. For hematological data, see supplemental Tables 1-3 and supplemental Figure 1.
The present study was performed on a small number of G6PD-deficient subjects that never consumed FBs before. Both elements may affect the study's strength. Yet, lack of any sign of oxidative RBC damage and hemolysis after ingestion of large amounts of raw high-V/C FBs may allow the conclusion that low-V/C FBs can be safely consumed by carriers of low-activity G6PD variants. Since ∼8000 to 10 000 years BC, FBs were cultivated and became a staple food in several semiarid regions.21 FBs are a hemolytic risk for >400 million G6PD-deficient men and women worldwide.5 Availability of low-V/C FBs with high protein, minerals, vitamins, and low fat content, well adapted to semiarid regions,22 may have a positive impact in agriculture and human nutrition particularly in poor countries hit by ever-increasing severe drought periods.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank volunteers for generous blood donations; B. Raffiot, Terres Inovia-France, for help with FB seed production; P. Dulieu, RDBIOTECH, Besançon, France, for help with V assay; and the doctors and staff of Alghero City Hospital for their help. P.A. thanks Lucio Luzzatto for helpful comments.
This work was supported in part by the Saskatchewan Pulse Crop Development Board (grant 0818/1007) (P.A.) and European Project on Faba Bean Breeding for Sustainable Agriculture (EUFABA) Project EU-Grant-QLRT-02307 (G.D. and P.A.).
The funders had no role in study design, data analysis, and preparation of the manuscript.
Authorship
Contribution: V.G., O.A.S., L.F.S., E.S., G.D., and P.A. designed research, analyzed data, and wrote the manuscript; P.A. had primary responsibility for the final text; and all authors conducted experiments and read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for O.A.S. is Wenner-Gren Institute, Stockholm University, Stockholm, Sweden.
Correspondence: Paolo Arese, Dipartimento di Oncologia, Università di Torino, Via Santena 5bis, 10126 Torino, Italy; e-mail: paolo.arese@unito.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal