In this issue of Blood, Lamy et al present the results of the LYSA/GOELAMS trial 02-03, where patients with limited-stage diffuse large B-cell lymphoma (DLBCL) received either 4 or 6 cycles of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) based on risk stratification, and those achieving complete response (CR) by positron emission tomography (PET) after 4 cycles either received 40 Gy of radiation or were observed. The outcomes were excellent regardless of radiation administration, which was not surprising because the study enrolled a favorable-risk cohort of patients. The role of radiation therapy (RT) is hard to discern in this setting.1
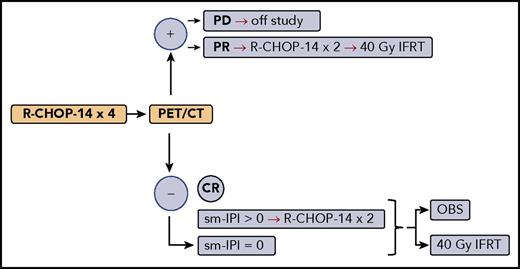
Simplified treatment schema for LYSA/GOELAMS trial 02-03. CT, computed tomography; IFRT, involved-field RT; OBS, observation; PD, progressive disease.
Simplified treatment schema for LYSA/GOELAMS trial 02-03. CT, computed tomography; IFRT, involved-field RT; OBS, observation; PD, progressive disease.
DLBCL presents as limited stage in ∼30% of cases. The standard of care was set by SWOG study S8736, which showed overall survival (OS) advantage with 3 cycles of CHOP chemotherapy closely followed RT over 8 cycles of CHOP.2 However, this survival advantage disappeared at 9 years, and 18-year follow-up of S8736 showed no difference in OS or progression-free survival, no significant difference in the rate of secondary malignancies, and persistent pattern of continuous relapse (also seen in a subsequent rituximab-containing study3 ), which is not seen in advanced-stage disease.4 S8736 also established the prognostic utility of a stage-modified (Miller et al2 ) international prognostic index (sm-IPI), which eliminated multiple extranodal sites from the standard IPI5 and dichotomized stage as 1 vs 2.
Significant variability in results in limited-stage DLBCL studies stems in large part from different definitions of limited stage, bulky disease, and risk stratification for patient selection. Most groups define limited-stage disease as nonbulky stage 1 or 2, usually without systemic symptoms and contained within a radiation field. However, SWOG has included bulky stage 1 and stage 1 with systemic symptoms in its limited-stage DLBCL trials, with no adverse outcome signal. SWOG has defined bulky disease as that in which the largest tumor diameter is ≥10 cm, whereas many groups have used more conservative definitions of 5, 7, or 7.5 cm. Risk assessment may be conducted using sm-IPI, IPI, or age-adjusted IPI.
Lamy et al report the results of the LYSA/GOELAMS (Lymphoma Study Association/French Acute Leukaemia and Blood Diseases West-East Group) trial 02-03, which enrolled 334 patients with stage 1/2 nonbulky DLBCL based on PET scan, with nonbulky defined as greatest tumor diameter <7 cm. Patients were randomly assigned upfront to receive either 40 Gy of involved-field RT or be observed, if they achieved CR based on PET scan after 4 cycles of R-CHOP administered every 14 days (R-CHOP-14). However, patients with sm-IPI >0 received an additional 2 cycles of R-CHOP-14 before proceeding with RT or observation. Also, patients who achieved only partial response (PR) received 2 more cycles of R-CHOP-14 and 40 Gy of RT regardless of upfront randomization (see figure). With median follow-up of 5.3 years, the outcomes were similarly spectacular regardless of RT: 5-year EFS of 89% vs 92% in the RT arm, and 5-year OS of 92% vs 96% in the RT arm.
The authors should be commended for conducting a large high-quality cooperative group study in a narrow subset of patients, which is why it took a long time to accrue (2005-2014). Because the French cooperative group pioneered the more intense regimen of rituximab plus doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (R-ACVBP) for higher-risk patients,6 the patients in this trial had favorable-risk DLBCL: 56% had sm-IPI of 0, 38% had sm-IPI of 1, and 64% were age <60 years. Therefore, the superb outcomes in this study should not come as a surprise.
The authors state that because 28 of 38 patients in PR achieved CR after additional chemotherapy and/or RT, they think that “PET-positive signals observed after cycle 4 were mainly related to residual lymphoma.”1 An alternative explanation would be that PET was detecting inflammation or effects of neutrophil growth factor use instead of active disease, which then could have resolved on its own. This has been reported particularly with R-CHOP-14, which requires tight PET scanning deadlines, with absolute majority of biopsies of PET+ patients showing no active lymphoma in 1 study.7 The fact that “the outcome of these PR patients did not differ from those reaching CR after 4 cycles of R-CHOP”1 could be used to argue that it was not in fact active lymphoma that PET was picking up, because patients who were truly refractory (ie, PR) to 4 cycles of R-CHOP should have had poor outcomes, which was clearly not the case.
Six cycles of R-CHOP as administered in advanced-stage disease remains a viable alternative to the shorter R-CHOP plus RT course, in part based on extrapolation from the MInT (MabThera International Trial) study, which enrolled a significant number of patients with limited-stage disease.8 Because the impact of RT after a full course of R-CHOP in nonbulky disease remains uncertain, the lack of impact of RT in this study is not surprising, since all patients achieving PR after 4 cycles of R-CHOP and those achieving CR but with sm-IPI of 1 received a total of 6 cycles of R-CHOP. Therefore, the 158 patients in CR with sm-IPI of 0 who were randomly assigned to RT or observation after only 4 cycles of R-CHOP constitute the true experimental arm. In this regard, the trial confirms the British Columbia Cancer Agency experience, where 1 additional cycle of R-CHOP was administered to patients who achieved CR on PET after 3 cycles of R-CHOP, for a total of 4, with OS >90%.9
In 2004, Miller10 published an editorial in which he outlined 3 risk groups based on SWOG S8736. The most favorable cohort (no bulk, sm-IPI of 0) had 5-year OS >90% regardless of treatment strategy, in the pre-rituximab era. A majority of patients in the study by Lamy et al belong to this group. So what is the take-home from this study? The only reasonable conclusion is that if you are such a patient (ie, in CR after 4 cycles of R-CHOP), RT may not be necessary. And thus, we arrive where we started.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal