Key Points
Ibrutinib has modest activity in FL with low response rates in rituximab-refractory patients.
CARD11 mutations predict for lack of response to ibrutinib.
Abstract
Most patients with follicular lymphoma (FL) experience multiple relapses necessitating subsequent lines of therapy. Ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor approved for the treatment of several B-cell malignancies, showed promising activity in FL in a phase 1 study. We report the results of a phase 2 trial evaluating ibrutinib in recurrent FL. Forty patients with recurrent FL were treated with ibrutinib 560 mg/d until progression or intolerance. The primary end point was overall response rate (ORR). Exploratory analyses included correlations of outcome with recurrent mutations identified in a cancer gene panel that used next-generation sequencing in pretreatment biopsies from 31 patients and results of early interim positron emission tomography/computed tomography scans in 20 patients. ORR was 37.5% with a complete response rate of 12.5%, median progression-free survival (PFS) of 14 months, and 2-year PFS of 20.4%. Response rates were significantly higher among patients whose disease was sensitive to rituximab (52.6%) compared with those who were rituximab refractory (16.7%) (P = .04). CARD11 mutations were present in 16% of patients (5 of 31) and predicted resistance to ibrutinib with only wild-type patients responding (P = .002). Maximum standardized uptake value at cycle 1 day 8 correlated with response and PFS. Ibrutinib was well-tolerated with a toxicity profile similar to labeled indications. Ibrutinib is a well-tolerated treatment with modest activity in relapsed FL. Evaluation of BTK inhibitors in earlier lines of therapy may be warranted on the basis of improved response rates in rituximab-sensitive disease. Somatic mutations such as CARD11 may have an impact on response to ibrutinib, may inform clinical decisions, and should be evaluated in larger data sets. This trial was registered at www.clinicaltrials.gov as #NCT01849263.
Introduction
B-cell receptor (BCR) signaling controls the differentiation and function of normal B cells. Dysregulation of the BCR pathway promotes the growth and survival of malignant B cells.1,2 Bruton tyrosine kinase (BTK), a critical enzyme in the BCR signaling cascade, phosphorylates phospholipase Cγ2 and stimulates downstream pathways essential for B-cell survival and proliferation.3,4 Ibrutinib is an irreversible, small-molecule inhibitor of BTK with efficacy in B-cell malignancies, including chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, lymphoplasmacytic lymphoma, marginal zone lymphoma, and mantle cell lymphoma.5-10
Follicular lymphoma (FL) cells exhibit enhanced BCR activation via both antigen-dependent and -independent mechanisms.11-13 In a phase 1 study of ibrutinib in relapsed B-cell malignancies, 6 (54%) of 11 patients with FL who received doses ≥2.5 mg/kg achieved an objective response.14,15 The median duration of response (DOR) and progression-free survival (PFS) were 12.3 and 13.4 months, respectively. Across all histologies, the overall response rate (ORR) was 60%. The recommended phase 2 dose was 560 mg/d, which was well tolerated and achieved full BTK occupancy in a range of body weights.
On the basis of encouraging phase 1 results, the phase 2 consortium conducted a trial of ibrutinib in patients with relapsed or refractory FL. In this trial, we demonstrated that response rates to ibrutinib were lower than previously reported for other B-cell malignancies, especially in patients refractory to prior rituximab. Additional goals were to correlate outcomes with baseline lymphoma mutations and with findings of early interim positron emission tomography/computed tomography (PET/CT) scans.
Materials and methods
Eligibility
Eligible patients were age 18 years or older, had histologically confirmed grade 1, 2, or 3A FL recurring after 1 or more chemotherapy regimens, and an Eastern Cooperative Oncology Group performance status ≤2. All patients had measurable disease ≥1.5 cm and were required to undergo a tumor biopsy at baseline. The following laboratory values were required: absolute neutrophil count ≥0.75 × 109/L, hemoglobin ≥8.0 g/dL, platelets ≥30 × 109/L, total bilirubin ≤1.5 × upper limit of normal, transaminases ≤2.0 × upper limit of normal, and creatinine clearance ≥30 mL/min. Patients who required warfarin or had a history of stroke or intracranial hemorrhage within 6 months, active transformed disease, central nervous system involvement, active infection, prior allogeneic stem cell transplantation, or prior BTK inhibitor treatment were not eligible. Inclusion criteria did not require that patients meet Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria or be symptomatic to enroll.
Study design and treatment
This multicenter, open-label study of ibrutinib in patients with recurrent FL was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review boards of each participating site. All patients provided written informed consent.
Patients were accrued at member institutions in the United States, Canada, and Singapore. Ibrutinib was administered at 560 mg once per day on continuous 28-day cycles until disease progression or unacceptable toxicity. Dose reductions were allowed in 140-mg increments to a minimum of 280 mg for adverse events (AEs). Ibrutinib was held and dose-reduced for treatment-related recurrent grade 4 neutropenia; grade 3 thrombocytopenia in the presence of significant bleeding; grade 4 thrombocytopenia; grade 3 to 4 nausea, vomiting, or diarrhea persisting despite antiemetic or antidiarrheal medication; and any other nonhematologic grade 4 or unmanageable nonhematologic grade 3 events.
Assessments
Response was assessed by using CT scans according to the 2007 Revised Response Criteria for Malignant Lymphoma.16 Restaging CT scans were performed on day 1 of cycles 3, 6, 9, and 12, and then every 6 cycles until the end of treatment. All patients treated in the United States underwent PET/CT with 18F-fluorodeoxyglucose (FDG) at baseline and cycle 3 day 1. PET/CT scans were not approved in Canada or Singapore. There was no central review of scans; PET/CT scans were graded visually in accordance with the Deauville criteria with scores of 4 or 5 considered positive and scores of 1, 2, or 3 considered negative.17 The primary end point was ORR by CT using the standard criteria outlined in both the Cheson 2007 and Lugano 2014 classification systems for CT-based response assessment. In patients who achieved a radiographic complete response (CR), a negative bone marrow biopsy was required if positive at baseline. Secondary end points included safety, overall survival (OS), time to response, DOR, PFS, and time to treatment failure (TTF). A correlative study of interim PET/CT parameters at cycle 1 day 8 and cycle 3 day 1 was performed in a subset of 20 patients treated at 1 center (Washington University) with a single observer (B.A.S.) performing quantitative assessment. Planned correlative biomarker analyses included lymphoma mutation profiling.
Statistical analysis
A one-stage binomial design with 36 evaluable patients provided 91% power to test the null hypothesis that the ORR is at most 20% vs the alternative hypothesis that the ORR is at least 40%, with a one-sided significance level of α = .09. At the final analysis, at least 11 responses were required in the first 36 evaluable patients to recommend further testing of this regimen in subsequent studies in this patient population. Four additional patients were enrolled to account for cancelled or ineligible patients for a total of 40 patients. All patients who received at least 1 dose of ibrutinib were considered evaluable for efficacy and safety. PFS was defined as the time from registration to progression or death. DOR was defined as the time from first documented response to the date of progression. TTF was defined as the time from registration to the date a decision was made to discontinue study treatment. The distributions of time to event measures were estimated by using the Kaplan-Meier method.
Recurrent mutations were evaluated for association with PFS, response, and DOR. Patient mutation data were analyzed on the basis of gene mutation status (mutated vs wild-type). Genes included were those mutated in ≥4 individuals (65 genes; supplemental Figure A, available on the Blood Web site). Analyses included time-to-event (log-rank statistics) and treatment response (Fisher’s exact test) associations (supplemental Methods).
The correlative study of exploratory PET/CT at cycle 1 day 8 and cycle 3 day 1 measured differences in maximum standardized uptake value (SUVmax) from baseline. The relationship with outcome was evaluated by using a univariable Cox proportional hazards model for PFS and Wilcoxon rank sum test for correlation with best response. A landmark analysis was used for estimating PFS from time points after baseline; log-rank statistics were used to assess differences between groups. Recursive partitioning algorithm methods were used in a model for PFS with cycle 1 day 8 SUVmax as a continuous variable to identify an optimal cutoff point for cycle 1 day 8 SUVmax based on our data.
Mutation analysis and sequencing
Thirty-one pre-ibrutinib tumor samples were sequenced. Nonmalignant DNA was not available for comparison. The targeted space was defined by the Personalis (Menlo Park, CA) Accuracy and Content Enhanced (ACE) Panel.18 The Genome Modeling System was used to count reference and variant alleles and to determine variant allele frequencies.19 A series of filters and manual review of selected variants was performed to create a high-quality list of 764 variants (179 genes) confirmed for analysis.20 Genomic visualizations were produced with GenVisR21 and ProteinPaint.22 Complete details of sample acquisition, data generation, and sequencing analysis are provided in supplemental Methods.
Results
Patients and treatment
From April 2013 to April 2014, 40 patients were enrolled and received at least 1 dose of ibrutinib (Table 1). Median age was 64 years, 50% of patients had a Follicular Lymphoma International Prognostic Index (FLIPI) score ≥3, and 62.5% met the GELF criteria for high tumor burden at study entry. Patients had a median of 3 prior regimens. Nearly half the patients were rituximab refractory, defined as having no objective response to a prior rituximab-containing regimen or relapse within 6 months of the last dose of rituximab, and 35% were refractory to their most recent chemotherapy regimen.
Baseline patient characteristics (N = 40)
| Characteristic . | No. . | % . |
|---|---|---|
| Median age, years (range) | 64 (46-82) | |
| Sex | ||
| Male | 28 | 70 |
| Female | 12 | 30 |
| FLIPI score | ||
| 0-1 (low risk) | 6 | 15 |
| 2 (intermediate risk) | 14 | 35 |
| 3-5 (high risk) | 20 | 50 |
| Stage | ||
| 1-2 | 7 | 17.5 |
| 3-4 | 33 | 82.5 |
| Bone marrow involvement | 18 | 45 |
| B symptoms | 4 | 10 |
| Elevated LDH | 10 | 25 |
| GELF criteria | 25 | 62.5 |
| FL grade | ||
| I or II | 34 | 85 |
| IIIA | 6 | 15 |
| Median No. of prior therapies (range) | 3 (1-11) | |
| Rituximab | 37 | 92.5 |
| Stem cell transplantation | 8 | 20 |
| Disease status | ||
| Refractory to most recent prior therapy* | 14 | 35 |
| Rituximab refractory* | 18 | 45 |
| Characteristic . | No. . | % . |
|---|---|---|
| Median age, years (range) | 64 (46-82) | |
| Sex | ||
| Male | 28 | 70 |
| Female | 12 | 30 |
| FLIPI score | ||
| 0-1 (low risk) | 6 | 15 |
| 2 (intermediate risk) | 14 | 35 |
| 3-5 (high risk) | 20 | 50 |
| Stage | ||
| 1-2 | 7 | 17.5 |
| 3-4 | 33 | 82.5 |
| Bone marrow involvement | 18 | 45 |
| B symptoms | 4 | 10 |
| Elevated LDH | 10 | 25 |
| GELF criteria | 25 | 62.5 |
| FL grade | ||
| I or II | 34 | 85 |
| IIIA | 6 | 15 |
| Median No. of prior therapies (range) | 3 (1-11) | |
| Rituximab | 37 | 92.5 |
| Stem cell transplantation | 8 | 20 |
| Disease status | ||
| Refractory to most recent prior therapy* | 14 | 35 |
| Rituximab refractory* | 18 | 45 |
LDH, lactate dehydrogenase.
Refractory status was defined as no response, disease progression, or relapse within 6 months of completing therapy.
The median number of cycles administered was 11 (range, 1-35 cycles). At a median follow-up of 25.5 months (range, 11.3-33.1 months) in patients still alive, 5 patients were still receiving ibrutinib at cycles 18 to 35. Reasons for treatment discontinuation included disease progression (n = 29), refusal (n = 2), death (n = 1), and AEs (n = 3). Median TTF was 10.0 months (95% confidence interval [CI], 6.0-16.2 months).
AEs and dose modifications
Table 2 lists all grade ≥3 AEs; 42.5% of patients experienced at least 1 grade 3 to 4 AE. Grade 3 to 4 neutropenia (10%), lymphopenia (7.5%), anemia (7.5%), infection (7.5%), and diarrhea (5%) each occurred in more than 1 patient. One patient experienced grade 2 hypertension, but there were no reports of grade 3 to 4 hypertension. Dose reductions occurred in 5 patients, most commonly for neutropenia. AEs led to treatment discontinuation in 3 patients (1 patient with aortic hematoma after cycle 10, 1 with supraventricular tachycardia with Takotsubo cardiomyopathy after cycle 30, and 1 with pneumonia during cycle 1). One death occurred on treatment in a patient with cryptogenic cirrhosis who died suddenly from a massive upper gastrointestinal hemorrhage during cycle 5.
Grade ≥3 AEs
| AE . | All attributions (N = 40) . | |||||
|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 5 . | ||||
| No. . | % . | No. . | % . | No. . | % . | |
| Neutropenia | 1 | 2.5 | 3 | 7.5 | ||
| Lymphopenia | 2 | 5 | 1 | 2.5 | ||
| Anemia | 3 | 7.5 | ||||
| Infection | 2 | 5 | 1 | 2.5 | ||
| Diarrhea | 2 | 5 | ||||
| Leukocytosis | 3 | 7.5 | ||||
| Thrombocytopenia | 2 | 5 | ||||
| Gastric hemorrhage | 1 | 2.5 | ||||
| Hypercalcemia | 1 | 2.5 | ||||
| Spleen disorder | 1 | 2.5 | ||||
| Cardiac disorders (other Takotsubo cardiomyopathy) | 1 | 2.5 | ||||
| Chest pain | 1 | 2.5 | ||||
| Supraventricular tachycardia | 1 | 2.5 | ||||
| Cataract | 1 | 2.5 | ||||
| Fatigue | 2 | 5.0 | ||||
| Hip fracture | 1 | 2.5 | ||||
| Hyperuricemia | 1 | 2.5 | ||||
| Hypokalemia | 1 | 2.5 | ||||
| Dyspnea | 1 | 2.5 | ||||
| Rash | 1 | 2.5 | ||||
| Skin ulceration | 1 | 2.5 | ||||
| Hematoma | 1 | 2.5 | ||||
| Thromboembolic event | 1 | 2.5 | ||||
| AE . | All attributions (N = 40) . | |||||
|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 5 . | ||||
| No. . | % . | No. . | % . | No. . | % . | |
| Neutropenia | 1 | 2.5 | 3 | 7.5 | ||
| Lymphopenia | 2 | 5 | 1 | 2.5 | ||
| Anemia | 3 | 7.5 | ||||
| Infection | 2 | 5 | 1 | 2.5 | ||
| Diarrhea | 2 | 5 | ||||
| Leukocytosis | 3 | 7.5 | ||||
| Thrombocytopenia | 2 | 5 | ||||
| Gastric hemorrhage | 1 | 2.5 | ||||
| Hypercalcemia | 1 | 2.5 | ||||
| Spleen disorder | 1 | 2.5 | ||||
| Cardiac disorders (other Takotsubo cardiomyopathy) | 1 | 2.5 | ||||
| Chest pain | 1 | 2.5 | ||||
| Supraventricular tachycardia | 1 | 2.5 | ||||
| Cataract | 1 | 2.5 | ||||
| Fatigue | 2 | 5.0 | ||||
| Hip fracture | 1 | 2.5 | ||||
| Hyperuricemia | 1 | 2.5 | ||||
| Hypokalemia | 1 | 2.5 | ||||
| Dyspnea | 1 | 2.5 | ||||
| Rash | 1 | 2.5 | ||||
| Skin ulceration | 1 | 2.5 | ||||
| Hematoma | 1 | 2.5 | ||||
| Thromboembolic event | 1 | 2.5 | ||||
Efficacy
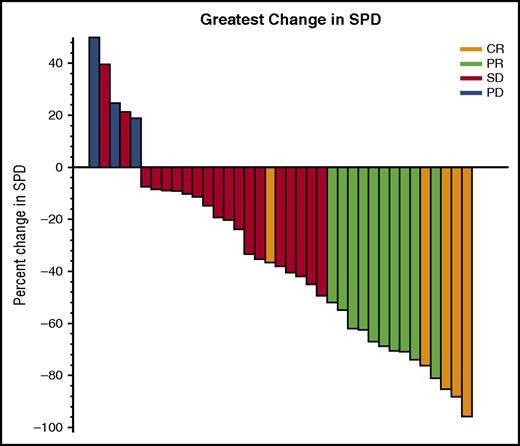
Five patients (12.5%) achieved CR and 10 (25%) achieved partial response (PR) by CT criteria for an ORR of 37.5% (95% CI, 22.7%-54.2%); the predefined criteria to conclude the regimen were met. In all, 47.5% patients had a best response of stable disease. Two patients were not assessed because of early discontinuation after 20 days on study as a result of pneumonia and hip fracture; both were considered treatment failures. Twenty-six patients completed PET/CT at cycle 3 day 1, and 4 were metabolic CRs. These 4 patients had best response of CR (1 patient), PR (2 patients), and stable disease (1 patient) by CT criteria. PET/CT was not required after cycle 3 day 1 and was not performed at the time CR was noted by CT criteria. Response rates were higher among patients with rituximab-sensitive disease (52.6%) compared with those who had rituximab-refractory disease (16.7%; P= .04) (Table 3). Chemotherapy-refractory patients had a lower ORR than chemotherapy-sensitive patients; the difference was not significant (28.6% vs 42.3%; P = .50). There was no correlation between ORR and FLIPI, GELF, or number of prior regimens; however, patients with low- or intermediate-risk FLIPI had a trend toward a higher response rate compared with high-risk FLIPI (50% vs 25%; P = .19). Median time to response was 4.6 months (range, 1.8-10.4 months). For patients achieving CR, the median time to response was 2.9 months (range, 1.8-4.6 months) with a median time to CR of 10.1 months (range, 4.6-21.3 months). Sixty percent of patients had a reduction in tumor volume of at least 20% (Figure 1). Response rates were not significantly different in patients receiving a starting dose ≥8.3 mg/kg of ibrutinib (1 [16.7%] of 6) compared with those who received a starting dose <8.3 mg/kg (14 [42.4%] of 33; P = .38) based on on-study weight.
Response rates by disease status
| Disease status . | No. . | CR . | PR . | Response rate (%) . | 95% CI . |
|---|---|---|---|---|---|
| Rituximab naïve | 3 | 1 | 1 | 66.7 | 9.4-99.2 |
| Rituximab sensitive | 19 | 3 | 7 | 52.6 | 28.9-75.6 |
| Rituximab refractory | 18 | 1 | 2 | 16.7 | 3.6-41.4 |
| Chemotherapy sensitive* | 26 | 4 | 7 | 42.3 | 23.4-63.1 |
| Chemotherapy refractory* | 14 | 1 | 3 | 28.6 | 8.4-58.1 |
| Disease status . | No. . | CR . | PR . | Response rate (%) . | 95% CI . |
|---|---|---|---|---|---|
| Rituximab naïve | 3 | 1 | 1 | 66.7 | 9.4-99.2 |
| Rituximab sensitive | 19 | 3 | 7 | 52.6 | 28.9-75.6 |
| Rituximab refractory | 18 | 1 | 2 | 16.7 | 3.6-41.4 |
| Chemotherapy sensitive* | 26 | 4 | 7 | 42.3 | 23.4-63.1 |
| Chemotherapy refractory* | 14 | 1 | 3 | 28.6 | 8.4-58.1 |
On the basis of response to most recent prior chemotherapy.
Maximum reduction in sum of perpendicular diameters (SPDs). Shown are the 37 patients who had follow-up scans available for bidimensional measurements. Two additional patients had no follow-up scans because of early withdrawal for pneumonia and refusal after hip fracture and were considered to be nonresponders for response rate determination. One patient had progressive disease but bidimensional measurements were not available for the new lesions. PD, progressive disease; SD, stable disease.
Maximum reduction in sum of perpendicular diameters (SPDs). Shown are the 37 patients who had follow-up scans available for bidimensional measurements. Two additional patients had no follow-up scans because of early withdrawal for pneumonia and refusal after hip fracture and were considered to be nonresponders for response rate determination. One patient had progressive disease but bidimensional measurements were not available for the new lesions. PD, progressive disease; SD, stable disease.
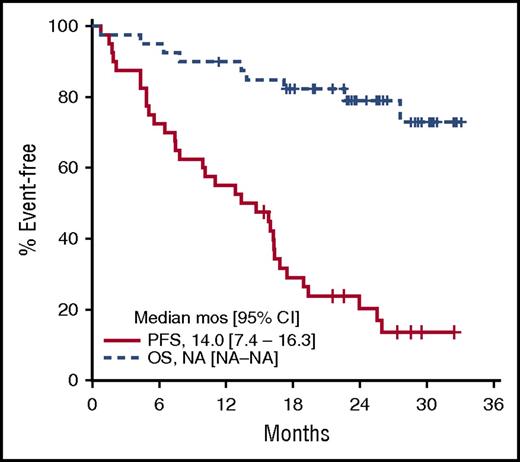
The median DOR was 13.9 months (95% CI, 5.8 months to not attained) and was not different for patients with PR vs CR. Five responders remain in remission, including 4 patients still on treatment. Thirty-one patients (78%) have progressed, and 9 patients have died (7 as a result of progressive disease, 1 as a result of pneumonia unrelated to treatment, and 1 as a result of gastric hemorrhage). The median PFS was 14.0 months (95% CI, 7.4-16.3 months; Figure 2) and the 2-year PFS was 20.4% (95% CI, 10.7%-38.6%). Median OS has not been reached, and the 2-year OS is 79.0% (95% CI, 67.0%-93.2%) (Figure 2).
Kaplan-Meier PFS and OS estimates. Survival analysis included 40 patients with evaluable disease completing at least 1 dose of ibrutinib. Median PFS was 14.0 months (95% CI, 7.4-16.3 months). Median OS was not reached. NA, not achieved.
Kaplan-Meier PFS and OS estimates. Survival analysis included 40 patients with evaluable disease completing at least 1 dose of ibrutinib. Median PFS was 14.0 months (95% CI, 7.4-16.3 months). Median OS was not reached. NA, not achieved.
Exploratory recurrent mutation analyses
Pre-ibrutinib lymphoma samples were available from 31 patients and were sequenced by using the Personalis ACE panel. Patients with CARD11 mutations (n = 5) had inferior response to ibrutinib therapy (Figure 3A), with only CARD11 wild-type patients obtaining a response (P = .002; Q = 0.054). Patients with CARD11 mutations also had inferior PFS compared with patients who were CARD11 wild-type (P = .02) (Figure 3B). The CARD11 mutations observed in this cohort occurred at or near several mutation hotspots previously reported in the Catalogue of Somatic Mutations in Cancer (COSMIC) (Figure 3C). Additional associations observed included IGLL5 mutations (n = 8) with improved PFS (P = .033) as well as KMT2D (n = 11; P = .031) and FOXO1 (n = 5; P = .007) mutations with longer DORs than those for wild-type patients (supplemental Table A). Complete results for mutations in recurrent genes are provided in supplemental Tables A and B and all variants are provided in supplemental Table C. In this sample set, all association testing was univariable and considered hypothesis generating.
Mutations in CARD11 are associated with inferior clinical outcomes after ibrutinib therapy in relapsed or refractory FL patients. (A) The distribution of best response stratified by CARD11 mutation status for patients with available pre-ibrutinib lymph node samples (n = 31). (B) PFS was significantly shorter in patients with CARD11 mutations (n = 5) compared with wild-type patients. (C) Observed CARD11 mutations (n = 8; top) and CARD11 mutations in the Catalogue of Somatic Mutations in Cancer (COSMIC; bottom) found in lymphoid neoplasms (n = 195). Amino acid positions are indicated by numbers within peptide representations. Exon boundaries are indicated by vertical dashed lines. Patients with mutations in the coiled-coil domain had an event in <12 months. Several mutations observed in relapsed or refractory FL patients examined in this study were previously cataloged in COSMIC (eg, G126D and D230N). The protein mutation diagram was created with ProteinPaint.22 HR, hazard ratio.
Mutations in CARD11 are associated with inferior clinical outcomes after ibrutinib therapy in relapsed or refractory FL patients. (A) The distribution of best response stratified by CARD11 mutation status for patients with available pre-ibrutinib lymph node samples (n = 31). (B) PFS was significantly shorter in patients with CARD11 mutations (n = 5) compared with wild-type patients. (C) Observed CARD11 mutations (n = 8; top) and CARD11 mutations in the Catalogue of Somatic Mutations in Cancer (COSMIC; bottom) found in lymphoid neoplasms (n = 195). Amino acid positions are indicated by numbers within peptide representations. Exon boundaries are indicated by vertical dashed lines. Patients with mutations in the coiled-coil domain had an event in <12 months. Several mutations observed in relapsed or refractory FL patients examined in this study were previously cataloged in COSMIC (eg, G126D and D230N). The protein mutation diagram was created with ProteinPaint.22 HR, hazard ratio.
Exploratory PET analysis
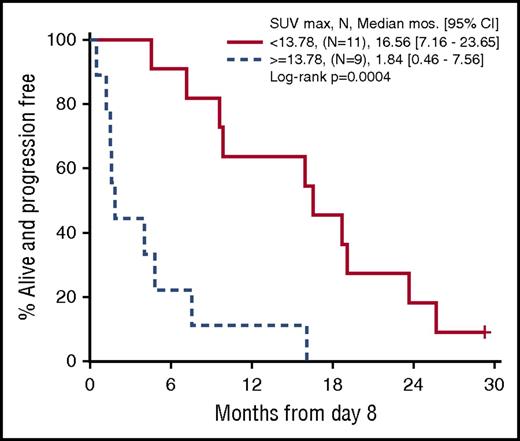
Twenty patients treated at a single institution, including 6 responders, had a PET/CT scan at cycle 1 day 8. These exploratory scans were performed with a uniform technique, and a single observer was responsible for quantitative assessment. At baseline, cycle 1 day 8, and cycle 3 day 1, SUVmax was defined as the most FDG-avid lesion irrespective of location, which parallels the 5-point scale (Deauville score) method. SUVmax as a continuous measure at cycle 1 day 8 correlated with both response (P = .03) and PFS (P = .03). Recursive partitioning algorithms identified a cutoff point of 13.78 for day 8 SUVmax that best predicted PFS. Figure 4 shows the PFS curve for patients whose day 8 SUVmax was ≥13.78 vs <13.78 (P = .0004). No significant association with PFS was seen with change in SUVmax (∆SUVmax) at cycle 1 day 8. In this subset of 20 patients, there was also no association between PFS and baseline SUVmax, or ∆SUVmax from baseline to cycle 3 day 1.
Progression-free survival by SUVmaxfrom cycle 1 day 8 PET/CT (n = 20). Patients were categorized above and below the cutpoint for SUVmax of 13.78 on day 8.
Progression-free survival by SUVmaxfrom cycle 1 day 8 PET/CT (n = 20). Patients were categorized above and below the cutpoint for SUVmax of 13.78 on day 8.
Discussion
In this small phase 2 trial, ibrutinib monotherapy exhibited modest antitumor activity in a heterogeneous group of patients with recurrent FL with an ORR of 37.5%, CR rate of 12.5%, and a median PFS of 14 months. Significantly lower response rates were observed for patients with rituximab-refractory disease. Ibrutinib administered at 560 mg once per day was well tolerated with a safety profile consistent with labeled indications.
The use of PET/CT to confirm response in FL is not an international standard of care and was not performed consistently in this trial because of lack of coverage for these studies. There were 3 patients who achieved a CR by PET/CT after cycle 2 but never achieved a CR by CT criteria. In addition, 4 of 5 patients with a CR by CT did not have a CR by PET. Whether the use of PET later in the course of treatment would increase the CR rate is unknown. Although not the primary end point, the median PFS of 14 months and the 2-year PFS of 20% are perhaps more useful than ORR in assessing the benefit of ibrutinib in FL.
The significantly lower response rate to ibrutinib in rituximab-refractory patients (16.7%) compared with rituximab-sensitive patients (52.6%) is intriguing. The mechanisms that underlie the concordance between FL patients responding to their last prior therapy (including rituximab) and a response to subsequent ibrutinib are unclear. Although ibrutinib is thought to act by directly disrupting BCR signals in lymphoma cells, additional functions, including changes in the immune system, have been reported.23 One possibility is that patients responding to rituximab have intact immune functionality compared with those who are refractory, and this in turn has an impact on ibrutinib-mediated immunomodulation. An alternative explanation is that patients who are refractory to rituximab-based therapy may be enriched for FLs with acquired genomic changes that are insensitive to ibrutinib, such as CARD11 mutations identified in this report. More speculative is the possibility that the direct apoptotic mechanisms of rituximab might overlap with ibrutinib-impacted signaling. Larger studies are needed to confirm this association between rituximab and ibrutinib resistance and ideally should include assessments of patient immune status and the FL genomic landscape to explore potential mechanisms.
The response rate and DOR to ibrutinib in FL were less than those reported for CLL, mantle cell lymphoma, and marginal zone lymphoma, perhaps because of a differential dependence on the BCR pathway (BTK) among different subtypes of B-cell malignancies. This has been demonstrated in diffuse large B-cell lymphoma (DLBCL) in which response rates to ibrutinib are higher in the activated B-cell phenotype (37%) compared with the germinal center phenotype (5%).24 Interestingly, Irish et al25 used phospho-flow cytometry to profile single cells within FL and discovered a subpopulation of cells with impaired BCR signaling. In that study, higher numbers of BCR-insensitive cells were present in relapsed compared with untreated FL.25 Despite high response rates in both untreated and relapsed CLL,5,6 prior lines of therapy may influence response to ibrutinib in FL. There was a trend in our study toward improved response rates in both rituximab-sensitive and chemotherapy-sensitive patients. Preliminary results of the DAWN study, a phase 2 study of ibrutinib in patients with chemoimmunotherapy-refractory FL, showed a significantly lower response rate of 20.9% with a median PFS of 4.6 months, a result which mirrors our finding of an ORR of 16.7% in rituximab-refractory FL and 28% in chemotherapy-refractory FL.26
Several studies are investigating first-line doublet and triplet regimens with ibrutinib in FL. A phase 2 trial of rituximab and ibrutinib reported an 82% ORR (CR, 27%).27 At a median follow-up of 10.2 months, the median PFS and DOR were not reached. Results of a 20-patient cohort treated with single-agent ibrutinib for 8 weeks before initiating rituximab have not yet been reported but may provide additional information about ibrutinib activity in untreated FL. Interpretation of rituximab-ibrutinib results in treatment-naïve FL are limited, given reported response rates of 77% to single-agent rituximab with CR rates of 37%.28 Two randomized trials of rituximab vs rituximab plus ibrutinib in untreated FL are ongoing (NCT0245111 and NCT02947347). A phase 1 trial of rituximab, lenalidomide, and ibrutinib in 22 previously untreated FL patients showed a response rate of 91% (CR, 63%), but the regimen was intolerable because of the high incidence of clinically significant rash.29
Despite studies that show complete BTK occupancy at doses of 2.5 mg/kg/d, higher doses were tested in the phase 1 study in B-cell lymphoma. The 8 FL patients who received high-dose ibrutinib, defined as ≥8.3 mg/kg/d, had higher response rates (ORR, 63%; CR, 38%) than the 8 patients in the low-dose (≤5 mg/kg/d) group (ORR, 25%; CR, 0%).27 Although the number of patients in our study receiving doses ≥8.3 mg/kg based on on-study weight was small (n = 6), there was no suggestion of a higher response rate in this subset, with only 1 (16.7%) of 6 responding.
We also explored the hypothesis that somatic genomic mutations present before the start of ibrutinib therapy may have an impact on responses. We observed a putative negative impact of CARD11 mutations on both response and PFS. Of the 8 CARD11 mutations, 5 were in the coiled-coil domain, which have been associated with constitutive activation of NF-κB signaling in cell lines.30 Patients with coiled-coil mutations had a shorter PFS (<12 months), consistent with the hypothesis that these CARD11 mutations constitutively activate NF-κB signaling downstream of BTK. However, functional genetic experiments will be required to experimentally define the NF-κB activation status for the observed CARD11 mutations. The correlation between CARD11 mutations and resistance to ibrutinib has also been reported in a phase 1/2 study of ibrutinib in relapsed DLBCL, with no responses among 3 patients with CARD11 mutations compared with 8 of 24 patients with wild-type CARD11 who responded.24
Additional recurrent mutations that warrant further study in larger numbers of ibrutinib-treated patients include IGLL5, which has previously been shown to be recurrently mutated in DLBCL31 and low-risk CLL.32 More broadly, these findings suggest that somatic mutations present in a lymphoma before treatment may have an impact on the response to a targeted therapy such as ibrutinib and could potentially be used to inform clinical decisions. It is worth noting that the known acquired ibrutinib resistance mutations previously identified in CLL were not detected in our FL samples.33-36 This suggests that although CLL and FL are both B-cell lymphomas, genomic heterogeneity exists between these malignancies that extends to differing mechanisms of resistance to BTK inhibitors. With larger FL data sets, prognostic models such as the clinicogenetic risk model containing 7 genes (M7-FLIPI), which integrates the gene mutation status of multiple genes (including CARD11) with clinical risk factors, may provide additional guidance.37
On the basis of the encouraging data regarding the prognostic significance of early interim FDG-PET/CT in Hodgkin lymphoma and DLBCL, we evaluated the prognostic significance of several PET parameters at baseline, day 8, and cycle 3 day 1. The decision to evaluate PET at day 8 was based on reports of the utility of very early PET with tyrosine kinase inhibitor therapy in other malignancies. For example, day 8 PET correctly predicted all CT responses to imatinib in gastrointestinal stromal tumors but preceded them by 7 weeks and response on day 8 was associated with longer PFS.38 In our study, SUVmax at day 8 as a continuous measure was associated with both response and PFS with a significant difference in PFS for patients with day 8 SUVmax <13.78 vs ≥13.78. Unfortunately, more clinically useful parameters such as SUVmax at baseline or ∆SUVmax did not correlate with response, perhaps because of the small sample size. Ideally, larger studies assessing the value of PET/CT during therapy with kinase inhibitors in FL may allow early intervention in patients who are unlikely to benefit. Further exploration of the utility of very early PET is warranted.
Second-generation BTK inhibitors in clinical development boast higher selectivity for BTK and consequently reduced toxicity compared with ibrutinib.39,40 A phase 1b study of acalabrutinib with or without rituximab is ongoing for patients with relapsed FL (NCT02180711). These more selective inhibitors may improve the therapeutic index, perhaps making them a better option for first-line or low-tumor-burden FL studies in which low toxicity treatments are appealing.
Although definitive conclusions on the utility of ibrutinib in FL are limited by the small size of our study, response rates to ibrutinib in relapsed FL do not seem to be as encouraging as those seen in other B-cell lymphomas and CLL. Higher response rates in patients with rituximab-sensitive disease may support assessment of the drug in earlier lines of therapy. The favorable AE profile in most patients, particularly the lack of myelosuppression, may be beneficial in patients who will require multiple future lines of therapy. Combinations with other agents must be approached with caution, given the unexpected toxicities seen with combinations including ibrutinib or idelalisib in previous studies.29,41 Our exploratory analysis of somatic mutations requires confirmation but suggests that exclusion of patients with CARD11 mutations from trials evaluating BTK inhibitors may be considered to enrich for responders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health Phase 2 Consortium grant N01-CM-2011-00099, by National Cancer Institute Cancer Center Support grant P30 K22CA188163 (Alvin J. Siteman Cancer Center Imaging and Response Assessment Core) and CA91842 (O.L.G.); by National Human Genome Research Institute grant K99HG007940 (M.G.); by the Larry and Winnie Chiang Fellowship (F.G.); by a Lymphoma Research Foundation Follicular Lymphoma Pathway Award (T.A.F.), and by Pharmacyclics LLC (research biopsies and gene sequencing).
The article content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: N.L.B., B.R.L., J.G.K., B.A.S., M.G., O.L.G., F.G., and T.A.F. contributed to conception and design of the study; and N.L.B., B.A.C., B.R.L., S.M.A., J.G.K., C.B.R., L.S.T., D.M.A., K.K., C.R., J.Q., B.A.S., M.G., O.L.G., F.G., and T.A.F. collected, assembled, analyzed, and interpreted data and helped write and provided final approval of the manuscript.
Conflict-of-interest disclosure: N.L.B. obtained research funding from Janssen Pharmaceuticals, Inc. and Pharmacyclics LLC. S.M.A. received an honorarium from WebMD and Research to Practice, research funding from Bristol-Myers Squibb, Celldex Therapeutics, Inc, Seattle Genetics, Merck, Affimed Therapeutics, and Trillium Therapeutics Inc. S.T.L. served as a consultant for Takeda Pharmaceuticals, Roche Holding AG, GlaxoSmithKline, Servier Laboratories, and Gilead Sciences and received research funding from Bayer. The remaining authors declare no competing financial interests.
Correspondence: Nancy L. Bartlett, Division of Oncology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8056, St. Louis, MO 63110; e-mail: nbartlet@wustl.edu.
References
Author notes
F.G. and T.A.F. are joint senior authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal